Does kraken live in the Kraken Sea? What forms of life could we find on Titan?
This is a translation of Paul Patton 's article published on www.universetoday.com .
Can life exist on the big satellite of Saturn, Titan? This question forces astrobiologists and chemists very carefully and creatively to understand the chemistry of life and how it could differ from other planets from the chemistry of life on Earth. In February, a team of researchers from Cornell University, including graduate student in chemical engineering James Stevenson, planetologist Jonathan Lunin and chemical engineer Paulette Clancy, published an innovative work, the essence of which is that living cell membranes can be formed in an exotic chemical environment present on this amazing companion.
In many aspects, Titan is the twin of Earth. This is the second largest satellite in the solar system, it is larger than the planet Mercury. Like the Earth, it has a dense atmosphere, whose pressure at the surface is slightly higher than on Earth. Apart from the Earth, Titan is the only object in our solar system, on the surface of which there are accumulations of fluid. NASA's Cassini spacecraft discovered an abundance of lakes and even rivers in the polar regions of Titan. The largest lake or sea, called the Kraken Sea, its area exceeds the area of the Caspian Sea on Earth. From the observations made by the spacecraft and the results of laboratory experiments, scientists found that in the atmosphere of Titan there are many complex organic compounds from which life is built.
')
Looking at all this, one may get the impression that Titan is an extremely habitable place. The name "Kraken", the so-called mythical sea monster, reflects the astrobiologists' secret hopes. But Titan is an alien twin of the Earth. It is almost 10 times farther from the sun than the Earth, its surface temperature is icy -180 degrees Celsius. As we know, water is an integral part of life, but on the surface of Titan it is as hard as stone. Water ice there is the same as rocks of silicon on Earth, forming the outer layers of the earth's crust.
The liquid that fills the lakes and rivers of Titan is not water, but liquid methane, most likely mixed with other substances such as liquid ethane, which are present on Earth in a gaseous state. If life is found in the seas of Titan, then it is not similar to our ideas about life. It will be a completely alien form of life for us, the organic molecules of which are not dissolved in water, but in liquid methane. Is it possible in principle?
A team from Cornell University has studied one key part of this difficult issue, considering the possibility of the existence of cell membranes in liquid methane. All living cells, in fact, is a system of self-sustaining chemical reactions, encased in a membrane. Scientists believe that cell membranes appeared at the very beginning of the history of life on Earth, and their formation, perhaps, was the first step towards the birth of life.
Here on Earth, everyone knows about cell membranes from a school biology course. These membranes are made up of large molecules called phospholipids. All phospholipid molecules have a “head” and a “tail”. The head is a phosphate group, where the phosphorus atom is bound to several oxygen atoms. The tail consists of one or several filaments of carbon atoms with a length of 15 to 20 atoms, to which are attached hydrogen atoms on each side. The head, due to the negative charge of the phosphate group, has an uneven distribution of electric charge, therefore it is called polar. The tail, on the other hand, is electrically neutral.

Such electrical properties of phospholipid molecules determine how they behave in aqueous solution. If we talk about the electrical properties of water, then its molecule is polar. The electrons in the water molecule are strongly attracted to the oxygen atom, rather than to two hydrogen atoms. Therefore, from the side of two hydrogen atoms the water molecule has a small positive charge, and from the side of the oxygen atom it has a small negative charge. Such polar properties of water force it to be attracted to the polar head of the phospholipid molecule, which is hydrophilic, and at the same time repel from non-polar tails, which are hydrophobic.
When phospholipid molecules dissolve in water, the combination of the electrical properties of both substances causes the phospholipid molecules to form a membrane. The membrane closes in a small sphere called a liposome. Phospholipid molecules form a bilayer two molecules thick. Polar hydrophilic molecules form the outer part of the membrane bilayer, which is in contact with water on the inner and outer surface of the membrane. Hydrophobic tails are connected to each other in the inner part of the membrane. Although the phospholipid molecules remain stationary relative to their layer, while their heads look outward and tails inward, the layers can still move relative to each other, giving the membrane sufficient mobility that is necessary for life.
Phospholipid bilayer membranes are the basis of all cell membranes on earth. Even by itself, a liposome can grow, reproduce itself, and facilitate the flow of certain chemical reactions necessary for the existence of living organisms. That is why some biochemists believe that the formation of liposomes was the first step towards the emergence of life. In any case, the formation of cell membranes should have taken place at the early stage of the origin of life on Earth.

If life on Titan exists in one form or another, be it a sea monster or (most likely) microbes, then they won't do without cell membranes, like all life on Earth. Can phospholipid bilayer membranes form in liquid methane on Titan? The answer is no. Unlike water, the electrical charge of a molecule of methane is distributed evenly. Methane does not have the polar properties of water, so it cannot attract the heads of phospholipid molecules. Such an opportunity is necessary for phospholipids to form the earth cell membrane.
Experiments were conducted in which phospholipids were dissolved in non-polar liquids at Earth's room temperature. Under such conditions, phospholipids form a "reverse" bilayer membrane. The polar heads of the phospholipid molecules are connected to each other in the center, attracted by their charges. Non-polar tails form the outer surface of the "reverse" membrane in contact with the non-polar solvent.

Can living organisms on Titan have a phospholipid reverse membrane? The Cornell team concluded that such a membrane is not suitable for life for two reasons. First, at cryogenic temperatures of liquid methane, the tails of phospholipids become rigid, thereby depriving the reverse membrane of any mobility necessary for the existence of life. Secondly, two key components of phospholipids - phosphorus and oxygen, most likely, are absent in methane lakes of Titan. In search of cell membranes that could exist on Titan, the Cornell team needed to go beyond the familiar biology school course.
Although phospholipid membranes were excluded, scientists believe that any cell membrane on Titan will still be similar to the phospholipid reverse membrane obtained in the laboratory. Such a membrane will consist of polar molecules connected to each other due to the difference in charge dissolved in non-polar liquid methane. What are these molecules? For answers, the researchers turned to the data obtained from Cassini and from laboratory experiments, during which the chemical composition of the atmosphere of Titan was recreated.
It is known that the atmosphere of Titan has a very complex chemical composition. It mainly consists of nitrogen and methane in a gaseous state. When the Cassini spacecraft analyzed the composition of the atmosphere by means of spectroscopy, it was discovered that there are traces of a wide variety of compounds of carbon, nitrogen and hydrogen in the atmosphere, called nitriles and amines. Researchers modeled the chemical composition of Titan’s atmosphere under laboratory conditions, exposing a mixture of nitrogen and methane to energy sources that mimic the sunlight on Titan. As a result, a broth of organic molecules, called tolines, was formed. They consist of compounds of hydrogen and carbon, that is, hydrocarbons, as well as nitriles and amines.
Researchers at Cornell University considered nitriles and amines potential candidates for the role of the basis for the formation of titanian cell membranes. Both groups of molecules are polar, which allows them to combine, thereby forming a membrane in non-polar liquid methane due to the polarity of the nitrogen groups that make up these molecules. They concluded that suitable molecules should be much less phospholipids, so that they can form mobile membranes at temperatures of the existence of methane in the liquid phase. They examined nitriles and amines containing chains of 3-6 carbon atoms. The groups containing nitrogen are called nitrogen groups, so the team gave the titanic analogue of the liposome the name “nitrogenosome”.
To synthesize azotosoma for experimental purposes is expensive and difficult, since experiments must be carried out at cryogenic temperatures of liquid methane. However, since the proposed molecules have already been well studied in other studies, the team at Cornell University considered it appropriate to turn to computational chemistry to determine whether the proposed molecules could form a mobile membrane in liquid methane. Computer models have already been successfully used to study the phospholipid cell membranes familiar to us.


Computer simulations conducted by our research team have shown that some substances can be eliminated, as they will not form a membrane, will be too rigid, or form solid substances. However, modeling has shown that some substances can form membranes with suitable properties. One of these substances was acrylonitrile, the presence of which in the atmosphere of Titan at a concentration of 10 ppm was discovered by Cassini. Despite the huge difference in temperatures between cryogenic azotosoma and liposomes that exist at room temperature, modeling has demonstrated that they have remarkably similar properties of stability and response to mechanical stress. Thus, cell membranes suitable for living organisms can exist in liquid methane.

Scientists from Cornell University consider the data obtained as a first step to demonstrate that life in liquid methane is possible, and to develop methods for detecting such life on Titan by future space probes. If life in liquid nitrogen is possible, then the following conclusions from this, go far beyond the boundaries of Titan.
In their search for habitable conditions in our galaxy, astronomers usually search for exoplanets whose orbits are within the star's habitability zone, which is determined by a narrow range of distances within which the temperature on the surface of the earth-like planet allows liquid water to exist. If life in liquid methane is possible, then the stars should also have a methane habitable zone - an area where methane on the surface of the planet or its satellite can be in the liquid phase, creating the conditions for the existence of life. Thus, the number of habitable planets in our galaxy will increase dramatically. Perhaps on some planets, methane life has evolved into complex forms that we can hardly imagine. Who knows, maybe some of them even look like sea monsters.
References:
N. Atkinson (2010) Alien Life on Titan? Hang on Just a Minute, Universe Today.
N. Atkinson (2010) Life on Titan Could be Smelly and Explosive, Universe Today.
ML Cable, SM Horst, R. Hodyss, PM Beauchamp, MA Smith, PA Willis, (2012) Titan tholins: Simulating Organic Chemicals, Chemical Reviews, 112: 1882-1909.
E. Howell (2014) Titan's Majestic Mirror-Like Lakes This Week, Universe Today.
J. Major (2013) Titan's North Pole is Loaded With Lakes, Universe Today.
CP McKay, HD Smith, (2005) Possibilities for methanogenic life, Titan, Icarus 178: 274-276.
J. Stevenson, J. Lunine, P. Clancy, (2015) Membrane, Science Advances 1 (1): e1400067.
S. Oleson (2014) Titan submarine: Exploring the depths of Kraken, NASA Glenn Research Center, Press release.
Cassini Solstice Mission, NASA Jet Propulsion Laboratory
NASA and ESA celebrate 10 years since Titan landing, NASA 2015

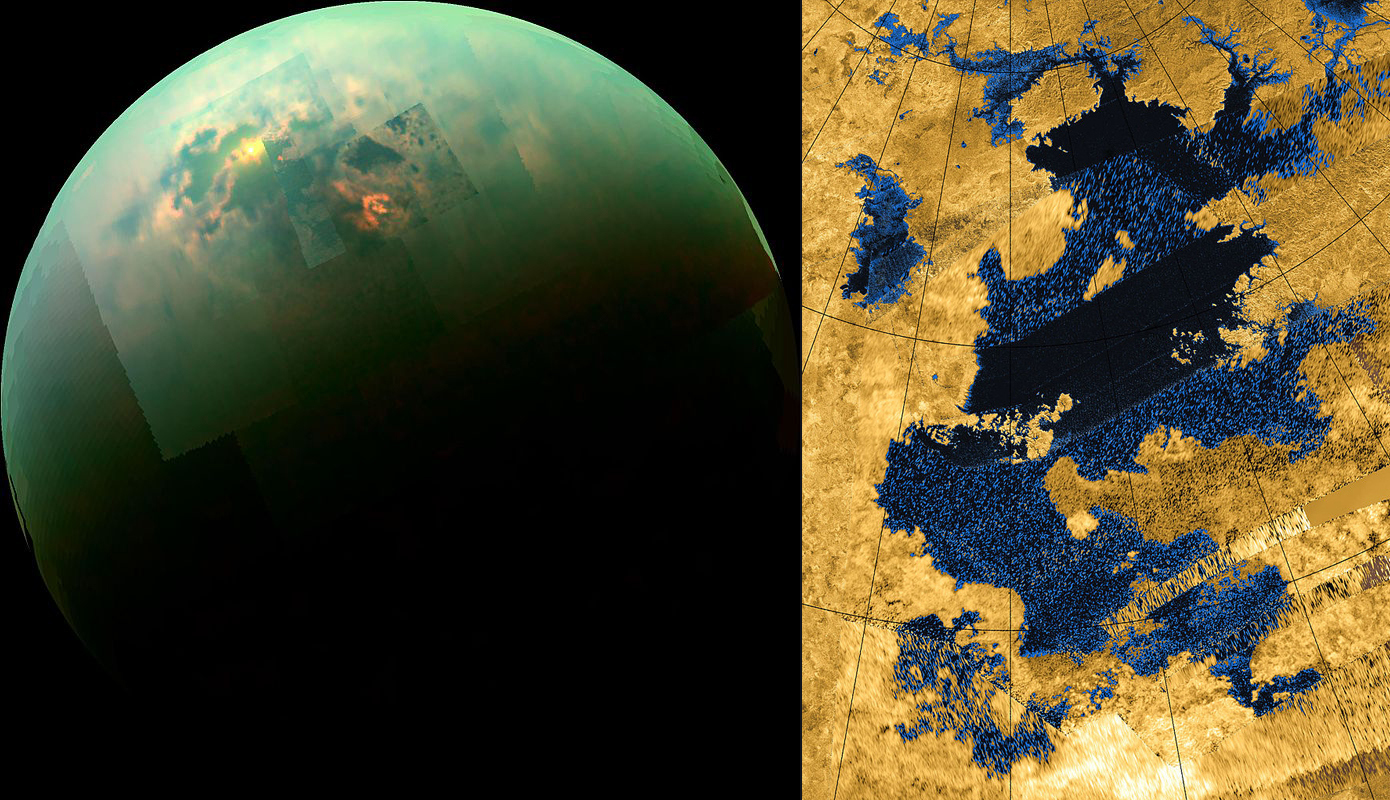
On the left side of the image you can see a mosaic of images taken by the Cassini spacecraft in the near infrared range. The picture shows the polar seas and the sunlight reflected from their surface. The reflection is located in the southern part of the Kraken Sea, the largest body of water on Titan. This reservoir is filled not with water at all, but with liquid methane and a mixture of other hydrocarbons. On the right side of the image you can see pictures of the Sea Kraken, taken by Cassini radar. Kraken is the name of a mythical monster who lived in the northern seas. Such a name seems to hint at what hopes astrobiologists associate with this mysterious alien sea.
Can life exist on the big satellite of Saturn, Titan? This question forces astrobiologists and chemists very carefully and creatively to understand the chemistry of life and how it could differ from other planets from the chemistry of life on Earth. In February, a team of researchers from Cornell University, including graduate student in chemical engineering James Stevenson, planetologist Jonathan Lunin and chemical engineer Paulette Clancy, published an innovative work, the essence of which is that living cell membranes can be formed in an exotic chemical environment present on this amazing companion.
In many aspects, Titan is the twin of Earth. This is the second largest satellite in the solar system, it is larger than the planet Mercury. Like the Earth, it has a dense atmosphere, whose pressure at the surface is slightly higher than on Earth. Apart from the Earth, Titan is the only object in our solar system, on the surface of which there are accumulations of fluid. NASA's Cassini spacecraft discovered an abundance of lakes and even rivers in the polar regions of Titan. The largest lake or sea, called the Kraken Sea, its area exceeds the area of the Caspian Sea on Earth. From the observations made by the spacecraft and the results of laboratory experiments, scientists found that in the atmosphere of Titan there are many complex organic compounds from which life is built.
')
Looking at all this, one may get the impression that Titan is an extremely habitable place. The name "Kraken", the so-called mythical sea monster, reflects the astrobiologists' secret hopes. But Titan is an alien twin of the Earth. It is almost 10 times farther from the sun than the Earth, its surface temperature is icy -180 degrees Celsius. As we know, water is an integral part of life, but on the surface of Titan it is as hard as stone. Water ice there is the same as rocks of silicon on Earth, forming the outer layers of the earth's crust.
The liquid that fills the lakes and rivers of Titan is not water, but liquid methane, most likely mixed with other substances such as liquid ethane, which are present on Earth in a gaseous state. If life is found in the seas of Titan, then it is not similar to our ideas about life. It will be a completely alien form of life for us, the organic molecules of which are not dissolved in water, but in liquid methane. Is it possible in principle?
A team from Cornell University has studied one key part of this difficult issue, considering the possibility of the existence of cell membranes in liquid methane. All living cells, in fact, is a system of self-sustaining chemical reactions, encased in a membrane. Scientists believe that cell membranes appeared at the very beginning of the history of life on Earth, and their formation, perhaps, was the first step towards the birth of life.
Here on Earth, everyone knows about cell membranes from a school biology course. These membranes are made up of large molecules called phospholipids. All phospholipid molecules have a “head” and a “tail”. The head is a phosphate group, where the phosphorus atom is bound to several oxygen atoms. The tail consists of one or several filaments of carbon atoms with a length of 15 to 20 atoms, to which are attached hydrogen atoms on each side. The head, due to the negative charge of the phosphate group, has an uneven distribution of electric charge, therefore it is called polar. The tail, on the other hand, is electrically neutral.

In our world, cell membranes are made up of phospholipid molecules dissolved in water. The basis of phospholipids are carbon atoms (gray), plus they also include hydrogen atoms (sky blue), phosphorus (yellow), oxygen (red) and nitrogen (blue). Due to the positive charge that the choline group containing the nitrogen atom and the negative charge of the phosphate group gives, the head of the phospholipids is polar and attracts water molecules. Thus, it is hydrophilic. The hydrocarbon tail is electrically neutral, therefore it is hydrophobic. The structure of the cell membrane depends on the electrical properties of phospholipids and water. Phospholipid molecules form a double layer — hydrophilic heads that are in contact with water outside, and hydrophobic tails look inward, merging with each other.
Such electrical properties of phospholipid molecules determine how they behave in aqueous solution. If we talk about the electrical properties of water, then its molecule is polar. The electrons in the water molecule are strongly attracted to the oxygen atom, rather than to two hydrogen atoms. Therefore, from the side of two hydrogen atoms the water molecule has a small positive charge, and from the side of the oxygen atom it has a small negative charge. Such polar properties of water force it to be attracted to the polar head of the phospholipid molecule, which is hydrophilic, and at the same time repel from non-polar tails, which are hydrophobic.
When phospholipid molecules dissolve in water, the combination of the electrical properties of both substances causes the phospholipid molecules to form a membrane. The membrane closes in a small sphere called a liposome. Phospholipid molecules form a bilayer two molecules thick. Polar hydrophilic molecules form the outer part of the membrane bilayer, which is in contact with water on the inner and outer surface of the membrane. Hydrophobic tails are connected to each other in the inner part of the membrane. Although the phospholipid molecules remain stationary relative to their layer, while their heads look outward and tails inward, the layers can still move relative to each other, giving the membrane sufficient mobility that is necessary for life.
Phospholipid bilayer membranes are the basis of all cell membranes on earth. Even by itself, a liposome can grow, reproduce itself, and facilitate the flow of certain chemical reactions necessary for the existence of living organisms. That is why some biochemists believe that the formation of liposomes was the first step towards the emergence of life. In any case, the formation of cell membranes should have taken place at the early stage of the origin of life on Earth.

On the left is water, a polar solvent consisting of hydrogen atoms (H) and oxygen (O). Oxygen attracts electrons more strongly than hydrogen, so the molecule on the side of hydrogen atoms has a positive net charge, and the oxygen side has a negative net charge. Delta (δ) denotes a partial charge, that is, less than a whole positive or negative charge. On the right is methane, the symmetrical arrangement of hydrogen atoms (H) around the central carbon atom (C) makes it a non-polar solvent.
If life on Titan exists in one form or another, be it a sea monster or (most likely) microbes, then they won't do without cell membranes, like all life on Earth. Can phospholipid bilayer membranes form in liquid methane on Titan? The answer is no. Unlike water, the electrical charge of a molecule of methane is distributed evenly. Methane does not have the polar properties of water, so it cannot attract the heads of phospholipid molecules. Such an opportunity is necessary for phospholipids to form the earth cell membrane.
Experiments were conducted in which phospholipids were dissolved in non-polar liquids at Earth's room temperature. Under such conditions, phospholipids form a "reverse" bilayer membrane. The polar heads of the phospholipid molecules are connected to each other in the center, attracted by their charges. Non-polar tails form the outer surface of the "reverse" membrane in contact with the non-polar solvent.

On the left, phospholipids are dissolved in water, in a polar solvent. They form a bilayer membrane, where the polar, hydrophilic heads face the water, and the hydrophobic tails face each other. On the right, phospholipids are dissolved in a non-polar solvent at Earth's room temperature, in such conditions they form a reverse membrane when the polar heads face each other, and the non-polar tails face outward to the non-polar solvent.
Can living organisms on Titan have a phospholipid reverse membrane? The Cornell team concluded that such a membrane is not suitable for life for two reasons. First, at cryogenic temperatures of liquid methane, the tails of phospholipids become rigid, thereby depriving the reverse membrane of any mobility necessary for the existence of life. Secondly, two key components of phospholipids - phosphorus and oxygen, most likely, are absent in methane lakes of Titan. In search of cell membranes that could exist on Titan, the Cornell team needed to go beyond the familiar biology school course.
Although phospholipid membranes were excluded, scientists believe that any cell membrane on Titan will still be similar to the phospholipid reverse membrane obtained in the laboratory. Such a membrane will consist of polar molecules connected to each other due to the difference in charge dissolved in non-polar liquid methane. What are these molecules? For answers, the researchers turned to the data obtained from Cassini and from laboratory experiments, during which the chemical composition of the atmosphere of Titan was recreated.
It is known that the atmosphere of Titan has a very complex chemical composition. It mainly consists of nitrogen and methane in a gaseous state. When the Cassini spacecraft analyzed the composition of the atmosphere by means of spectroscopy, it was discovered that there are traces of a wide variety of compounds of carbon, nitrogen and hydrogen in the atmosphere, called nitriles and amines. Researchers modeled the chemical composition of Titan’s atmosphere under laboratory conditions, exposing a mixture of nitrogen and methane to energy sources that mimic the sunlight on Titan. As a result, a broth of organic molecules, called tolines, was formed. They consist of compounds of hydrogen and carbon, that is, hydrocarbons, as well as nitriles and amines.
Researchers at Cornell University considered nitriles and amines potential candidates for the role of the basis for the formation of titanian cell membranes. Both groups of molecules are polar, which allows them to combine, thereby forming a membrane in non-polar liquid methane due to the polarity of the nitrogen groups that make up these molecules. They concluded that suitable molecules should be much less phospholipids, so that they can form mobile membranes at temperatures of the existence of methane in the liquid phase. They examined nitriles and amines containing chains of 3-6 carbon atoms. The groups containing nitrogen are called nitrogen groups, so the team gave the titanic analogue of the liposome the name “nitrogenosome”.
To synthesize azotosoma for experimental purposes is expensive and difficult, since experiments must be carried out at cryogenic temperatures of liquid methane. However, since the proposed molecules have already been well studied in other studies, the team at Cornell University considered it appropriate to turn to computational chemistry to determine whether the proposed molecules could form a mobile membrane in liquid methane. Computer models have already been successfully used to study the phospholipid cell membranes familiar to us.

It was found that acrylonitrile can be a possible basis for the formation of cell membranes in liquid methane on Titanium. It is known that it is present in the atmosphere of Titan at a concentration of 10 ppm, plus it was synthesized in the laboratory when simulating the effect of energy sources on Titan's nitrogen-methane atmosphere. Since this small polar molecule is capable of dissolving in liquid methane, it is a candidate for the role of a compound that can form cell membranes in terms of alternative biochemistry on Titanium. Blue - carbon atoms, blue - nitrogen atoms, white - hydrogen atoms.

The polar molecules of acrylonitrile line up in chains by the heads to the tails, forming membranes in non-polar liquid methane. Blue - carbon atoms, blue - nitrogen atoms, white - hydrogen atoms.
Computer simulations conducted by our research team have shown that some substances can be eliminated, as they will not form a membrane, will be too rigid, or form solid substances. However, modeling has shown that some substances can form membranes with suitable properties. One of these substances was acrylonitrile, the presence of which in the atmosphere of Titan at a concentration of 10 ppm was discovered by Cassini. Despite the huge difference in temperatures between cryogenic azotosoma and liposomes that exist at room temperature, modeling has demonstrated that they have remarkably similar properties of stability and response to mechanical stress. Thus, cell membranes suitable for living organisms can exist in liquid methane.

Simulation using computational chemistry shows that acrylonitrile and several other small polar organic molecules containing nitrogen atoms can form azotomes in liquid methane. Azotosomes are small, sphere-shaped membranes resembling liposomes formed from phospholipids dissolved in water. Computer simulation shows that acrylonitrile-based azotosomas will be both stable and flexible at cryogenic temperatures in liquid methane, which gives them the necessary properties to function as cell membranes for hypothetical titanian living organisms or any other organisms on the planet with liquid methane on the surface . The azotosome in the image has a size of 9 nanometers, which is approximately the size of the virus. Blue - carbon atoms, blue - nitrogen atoms, white - hydrogen atoms.
Scientists from Cornell University consider the data obtained as a first step to demonstrate that life in liquid methane is possible, and to develop methods for detecting such life on Titan by future space probes. If life in liquid nitrogen is possible, then the following conclusions from this, go far beyond the boundaries of Titan.
In their search for habitable conditions in our galaxy, astronomers usually search for exoplanets whose orbits are within the star's habitability zone, which is determined by a narrow range of distances within which the temperature on the surface of the earth-like planet allows liquid water to exist. If life in liquid methane is possible, then the stars should also have a methane habitable zone - an area where methane on the surface of the planet or its satellite can be in the liquid phase, creating the conditions for the existence of life. Thus, the number of habitable planets in our galaxy will increase dramatically. Perhaps on some planets, methane life has evolved into complex forms that we can hardly imagine. Who knows, maybe some of them even look like sea monsters.
References:
N. Atkinson (2010) Alien Life on Titan? Hang on Just a Minute, Universe Today.
N. Atkinson (2010) Life on Titan Could be Smelly and Explosive, Universe Today.
ML Cable, SM Horst, R. Hodyss, PM Beauchamp, MA Smith, PA Willis, (2012) Titan tholins: Simulating Organic Chemicals, Chemical Reviews, 112: 1882-1909.
E. Howell (2014) Titan's Majestic Mirror-Like Lakes This Week, Universe Today.
J. Major (2013) Titan's North Pole is Loaded With Lakes, Universe Today.
CP McKay, HD Smith, (2005) Possibilities for methanogenic life, Titan, Icarus 178: 274-276.
J. Stevenson, J. Lunine, P. Clancy, (2015) Membrane, Science Advances 1 (1): e1400067.
S. Oleson (2014) Titan submarine: Exploring the depths of Kraken, NASA Glenn Research Center, Press release.
Cassini Solstice Mission, NASA Jet Propulsion Laboratory
NASA and ESA celebrate 10 years since Titan landing, NASA 2015
Source: https://habr.com/ru/post/368557/
All Articles