Electrostatic cleaning of indoor air from the decay products of radon. Part 1, introduction
This publication addresses the problem of air pollution from radon and its daughter decay products ( DPR ). Various facts are collected and shown when radon is concentrated and can be detected by a household radiometer. An indication of the danger of radon in the west is indicated and a brief insight into the theory of radioactive decay and tissue damage is given. And finally, one idea has been proposed that has not yet been used for the purpose of air purification from DPR.
Radon is a radioactive gas, 7 times heavier than air, odorless and colorless in trace amounts, which are present everywhere on our planet. However, trace amounts are different ...
I first met radon, or rather its decay products 15 years ago, by chance, not knowing what it was and why. Conducting aimless experiments with a household radiometer, I repeatedly noticed that the CRT screen has an increased radioactive background.
')

It was noteworthy that this background was available on a television screen that had worked for some time. There was no activity on the TV that was just turned on or off for a long time.

Of course, the usual explanations for such a phenomenon are X-rays from the tube itself during operation or interference from electrostatics. But soft and very insignificant X-ray radiation cannot overcome the kinescope glass or be fixed by the sensor of the used dosimeter-radiometer.

And electrostatics should have a similar effect when turned on, and its effect can be eliminated by slightly moistening the glass. The next time radon manifested itself in fresh rainwater.

The radioactive background of the first raindrops is noticeably elevated and is quite observable with a household appliance. Few sources give a correct interpretation of this phenomenon. In our region (the city of Gomel), it is generally easy to blame for the clouds from the Chernobyl nuclear power plant (and then suddenly forest fires or the sarcophagus collapsed a little again - there are enough reasons to suspect). Although the "write off" of this is certainly not worth it.

Still, if you find a good place (mines, caves, basements), you can directly observe an increased background in the pits or caverns. This background is quickly normalized when airing the cavity being measured.

In general, radon is responsible for the increased background, clearly visible in the cases described. More precisely - the daughter products of its decay or DPR. Usually, without the so-called “special” equipment, radon has almost no chance of detecting.

True, such equipment is not always required to be delivered to the house. For example, in the United States there is a program for remote primary screening of radon in residents' homes. It is based on the exposure in the study area of the “Radon test kit” sponge [1] and then sending it to the research center.

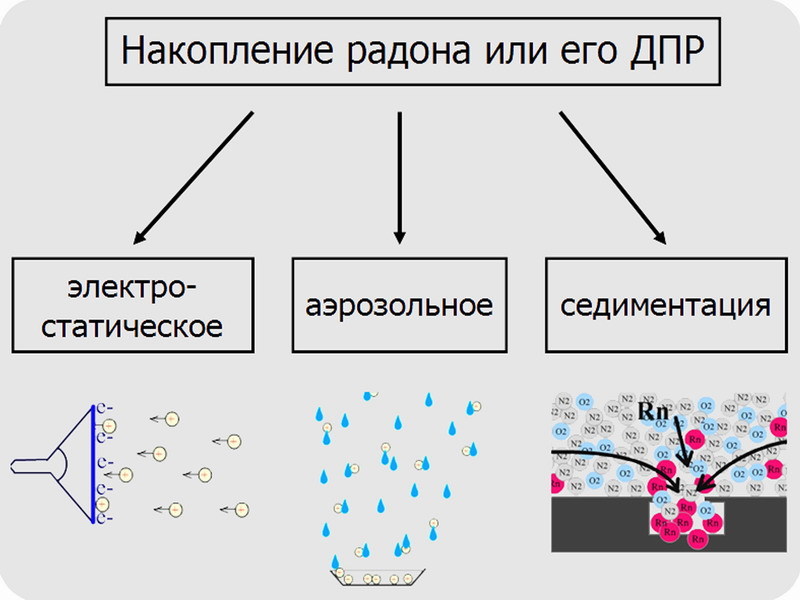
And so, the activity of radon itself and part of its decay products is not fixed by most measuring instruments, especially household ones. Only some products are visible, whose share is small and becomes sufficient for measurement only when conditions for their concentration are created. The three examples given are those cases: electrostatic accumulation of a TV, aerosol rain and sedimentation into a pit (density differences).
But where did radon come from? Sometimes the right answer to such a question is from everywhere. For if there is so much radon, then its source must be solid. After all, radon, like its daughter products, does not live for a long time - it disintegrates. In general, this issue has long been studied. Radon is also a daughter product of decay in a long row, usually led by 238th uranium. Or 232nd thorium.

Radon's immediate ancestor is radium, which is reflected in the name "radon". Uranium and thorium are present in rocks, soil, water, and of course in building materials. The average content or clarke of uranium is 27 ÷ 100000 percent, thorium - 96 ÷ 100000 [2].

In varying degrees, there will be all descendants next to the head of the series, the number of decays of each of which will be equal to the number of decays of the head. That is, for hundreds of thousands of years, uranium will have time (and it was already long ago!) To accumulate everything, including radium. But radon in a series of decays is special, because it is the only gas. He is able to leave the parent to migrate heavily.
Some minerals, such as the widely known granite, contain more uranium, up to 10-20 grams per ton. Normal sand - about a gram per ton.

It turns out that almost any building material is capable of emitting radon. This means that the basement is not profitable, not cleaner cave. In Europe and the USA, they are already seriously concerned [3] with the problem of radon - they are trying to solve it in the following ways: through preliminary inspection and non-use of materials and places emitting radon for construction purposes; installing forced ventilation in places of direct radon emission. In the US alone, this translates into a billion dollars in annual costs.

Why is it that radon should be considered more dangerous if its source will have a total activity 10 times higher, and the radon itself also constantly evaporates due to gas formation? To explain this, you need to talk a little bit about radioactive radiation in general. Another school course in physics says that radioactive rays are alpha, beta and gamma.
Actually, of course, there are more varieties, but these are the main ones. Gamma rays are electromagnetic radiation, by nature the same as radio waves or ordinary light, but of a “high” energy level (a thousand or more times higher).
This means that one portion or quantum of gamma rays will transmit as much energy as one thousand portions of ordinary light, and sometimes a million, billion. And in the nano-world of quantum mechanics, quantity does not mean quality: the fact that beyond a million portions of light is available to one portion of gamma rays. Rays obtained artificially or having a low level of energy are called x-rays. Beta and alpha rays are different - a stream of very fast particles.
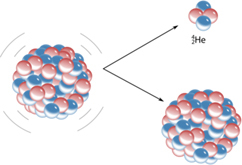
Beta rays - a stream of ordinary electrons, light particles with a negative charge. Alpha rays are helium atoms, devoid of all electrons, that is, their nuclei with a mass of 7300 times the mass of an electron. Charged positively.
The ability of rays to penetrate objects and the power of damage (for example, biological) inflicted by them is directly related to these factors. So, gamma rays are the most penetrating, beta rays are worse. And alpha rays almost do not overcome the dense barriers, even a small layer of air slows them down well.
The biological effect is the opposite. If we take for 1 damage caused by gamma, then beta also has 1, but alpha has a coefficient of 20 [4]. The alpha particle is heavy and large; it quickly dissipates its energy in matter. One particle, prior to its transformation into an ordinary helium atom, disrupts the electron layers of the atoms encountered, thereby destroying any chemical bonds.
One can imagine that at one moment in the volume of a living organism the size of a cubic nanometer appears a lot of random aggressive chemical compounds called free radicals. Each of which can damage another complex molecule that survived the first attack of the alpha particle. For example, DNA macromolecule. Theoretically, one alpha particle is enough to damage a single cell: sterilization or, worse, mutation.
The radons 220 and 222 decay, releasing the alpha particle and polonium. On the decay of half of radon need a little less than four days. Polonium, in turn, also decays, giving a beta particle. And so on the chain to lead.
As a result, wherever there is a source of radon, where there is radon itself and its daughter products, gamma, beta, and alpha radiation are present. In summary, you might think that only gamma rays or beta can deliver real harm. And this is true when it comes to conventional sources, since the upper dead layer of human skin does not transmit alpha rays to living cells and cannot be damaged by itself.
But we remember that radon is a gas. In addition, the product of its decay, although not gas, but is born in the air, in a monatomic form. Like the next nuclides, it will be very fine dust. Gas and dust can get into parts of the body where there is no protective layer - the airways and lungs. They implement the entire 20-fold damage from alpha rays plus a portion of the beta and gamma.
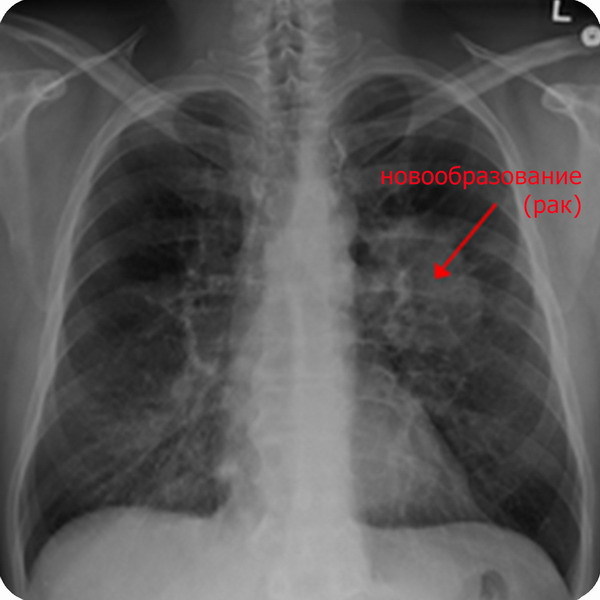
According to a report by the World Health Organization, indoor air pollution by radon causes lung cancer in the region from 3 to 14% of cases, which ranks second in frequency after smoking [5] [6].
Given that 1.5 million people die of lung cancer each year, among them 150,000 cases are caused by radon. Thus, among non-smoking radon on carcinogenicity comes out on top. A person breathing gas in a gas near a factory or avenue is less likely to develop lung cancer than a person who breathes only fresh air but lives or works in rooms where the earth or building materials do not noticeably emit a radioactive gas without color or smell.
How to solve the problem of radon? Before you solve a problem, study it. In our case, the study of the problem prompted one of its solutions, although not entirely directly. For radon radiometry, it is required to concentrate this gas in the air and use an alpha-sensitive radiometer. The traditional way is to push a large volume of air through a special filter and measure the alpha activity of the filter by the radiometer mentioned above.
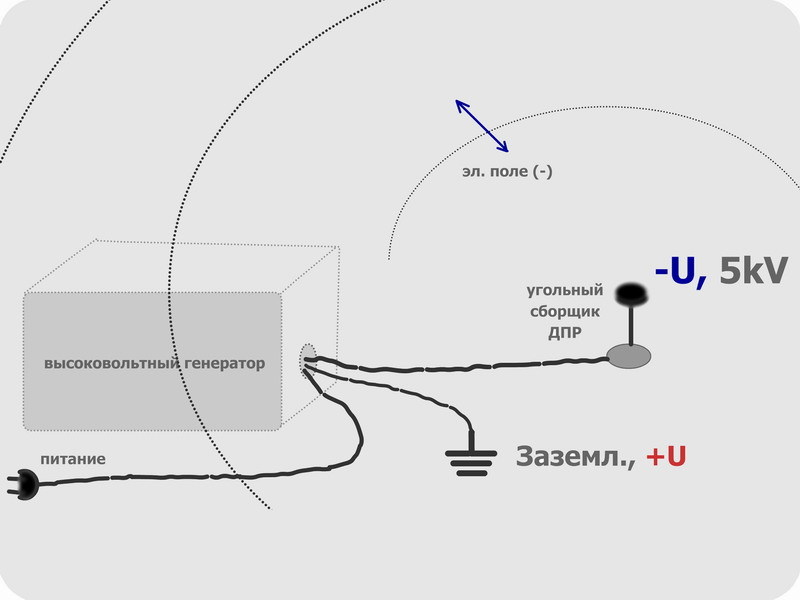
An alternative method is indirect, through the measurement of beta and gamma activity of the daughter decay products [7]. Electrostatics works here, because the decay products of radon are the nuclei of atoms with a significant positive charge. Of course, having lost speed, they will take away some of the electrons from the air or ordinary dust particles, sticking to the latter with the formation of an aerosol. However, a positive charge which will remain. Imagine that we have an electrode charged negatively. An electric field will spread around it, and when it comes under its action, positively charged dust particles will rush to the source of the field - on the electrode.
Thereby, DPR will be collected on a small area, where their decays are easier to accurately measure. And here we thought, is it possible to use the effect for another purpose - cleaning. After all, this is quite logical: since we took the DPR from the nearest volume of air, then it will take time until radon restores their concentration. By the way, even the newly formed radon itself will have a charge, hence it will be captured.
Currently, in order to identify the effectiveness of electrostatic accumulation of DPR in relation to cleaning, we are conducting research. We have already collected and tested cumulative installation.
The results of this setup were subjected to radiospectrographic analysis.
The analysis revealed gamma and beta active DPR of radon, although it is not surprising that the spectrograph revealed them. Even a household dosimeter-radiometer in the gamma measurement mode will record 5 times more decay events than the background, and in the beta + gamma mode - 50 times.
Of course, this is if the accumulation was sufficient time in the "infected" room.
The research facility itself consists of a high-voltage source and a precast electrode, in the role of which an activated carbon tablet acts. Activated carbon and so absorbs heavy vapors and gases, under charge, this property is multiplied.
The electrical circuit is still very simple:
Before developing a commercially efficient installation that solves a problem with radon, we still have to solve a number of problems, scientific and technical. For example, to identify how much better it is to spray the air with water. Or to prove the effectiveness of quantitative, in the context of the official scientific methodology. Find out the optimal voltage value, the shape and location of the electrodes, their number for minimal energy consumption, cost and convenient design in the form of a household appliance.
The project at the current stage is far from a ready-made solution, but not just an idea. It works.
Used sources of information:
- Radon Test Kit .
- Clark numbers of elements . Wikipedia.
- WHO handbook on indoor radon: a public health perspective / edited by Hajo Zeeb, and Ferid Shannoun. World Health Organization, 2009.
- Dosimetry in nuclear medicine .
- A. Demkin. Radon radioactive soil gas indoors and the risk of lung cancer .
- Lung cancer . Wikipedia.
- Mahdi Mohamed Ramdan. Radiometry of radon exhalation from building materials: dissertation dis. Candidate of Technical Sciences: Minsk, 1995.
The authors of the project Dzmitry Kalesnikau and Ivan Krauchanka have already spoken with this theme on February 6 at the Party Hard Conference ! 2016 in Minsk. Work on the project is ongoing ...
Source: https://habr.com/ru/post/368041/
All Articles