Ask Ethan # 21: Why does life exist?
In a nutshell, I can formulate everything that I learned about life: it continues.
- Robert Frost
The reader asks:
I would like to know your opinion on the application of the second law of thermodynamics to the origins of life.
And so, what he says .
It has long been known that at the beginning of the universe there was nothing like life. Not that complex multicellular life forms like mammals or insects - right after the Big Bang there were not even atoms! But from this very hot and dense soup of prehistoric matter and radiation everything gradually came out. And some of these steps are known to us completely.
')
Early quarks and gluons, part of the prehistoric plasma in the Universe, turned out to be tightly connected with each other when the Universe expanded and cooled. So there were protons and neutrons with which we are much better acquainted.
And for this there were reasons - it is energetically more preferable to have quarks and gluons bound in the form of protons and neutrons than to leave them in any other form. In an atmosphere of abundance of energy, nothing forces you to move to a more stable state - but as the Universe cools down, everything starts rolling downhill, and this process continues.
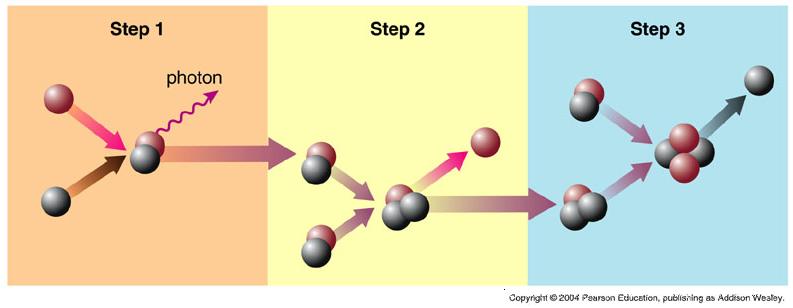
And when the Universe cools sufficiently so that the photons do not break more complex compounds of protons and neutrons, these compounds begin to form heavier and more stable atomic nuclei. If they had even more energy, they would have formed even heavier stable elements.
At that time, helium-4 was a practical limitation, but as the universe expanded and cooled, another way emerged in which particles could communicate with each other — the nuclei of atoms and electrons could make up the first neutral atoms.
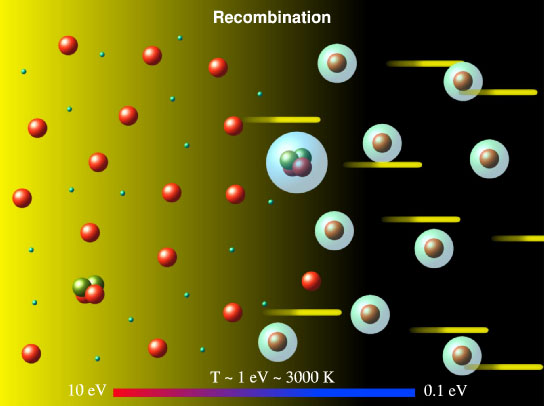
Of course, this could happen only when the Universe has cooled sufficiently; if you have too much energy, the neutral atom will be broken apart into ions and electrons. But if the surrounding radiation / temperature / energy of the particles becomes less than the energy that binds the electrons to the nucleus, you will get neutral atoms.
You, probably, have already noticed the principle: some processes occur spontaneously, but when the energy of the environment drops below a certain level, everything starts to come to a configuration with a lower energy state, and some processes require activation energy to cross over this level. As a result, everything comes to a more stable state. Let's go even further.

After gravity binds the universe into small pieces (again we see an example of spontaneous binding), the densest parts collect enough matter at a sufficiently high temperature and density to trigger nuclear fusion, which will produce ever heavier elements. Another example of the need for activation energy - but in the end we get even more strongly bonded products than the reagents we started with.
After the heaviest stars turn into supernovae, interstellar matter becomes enriched with all atoms of the periodic table, and they bind together in different ways and create molecules - first simple, but, after all, quite complex.
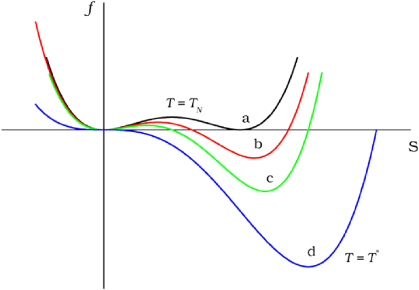
What needs to be remembered is that at every step we come to an increasingly energetically beneficial configuration, and there are always two ways — either we spontaneously roll down an energy hill, or we roll up to descend into a lower valley.
In all cases, the energy reactions look like one of these curves.
What about the big event? You can go from subatomic particles to particles that make up atoms, from protons, neutrons and electrons to atoms, from atoms to elements of the Periodic Table, from elements to various molecules, but at some point you need to jump. You know which one is from lifeless to life.

It is believed that either life originated long ago on Earth, or in space, even earlier than ours. And after this, Darwinian evolution — mutations, limited resources, and natural selection — will take us from the simplest self-replicating organisms to the full diversity of life that is currently observed on the planet.

But the mechanisms of this process this explanation does not determine.
In general, it is quite difficult to draw a line between life and lifeless. It will be amazing for my peers to find out how close the song from the show “Sesame Street” came to this definition: “breathe, eat and grow - so you know that you live”.
Life is separated from lifeless not by one characteristic, but by their set. Many inanimate things have some properties of the living. The same self-reproduction, for example.

The crystals are obviously not alive, but the crystalline structures reproduce themselves. As well as self-replicating microspheres at the molecular level.
And what is even more surprising - and turbulence, the simplest example of the flow of a liquid or gas.
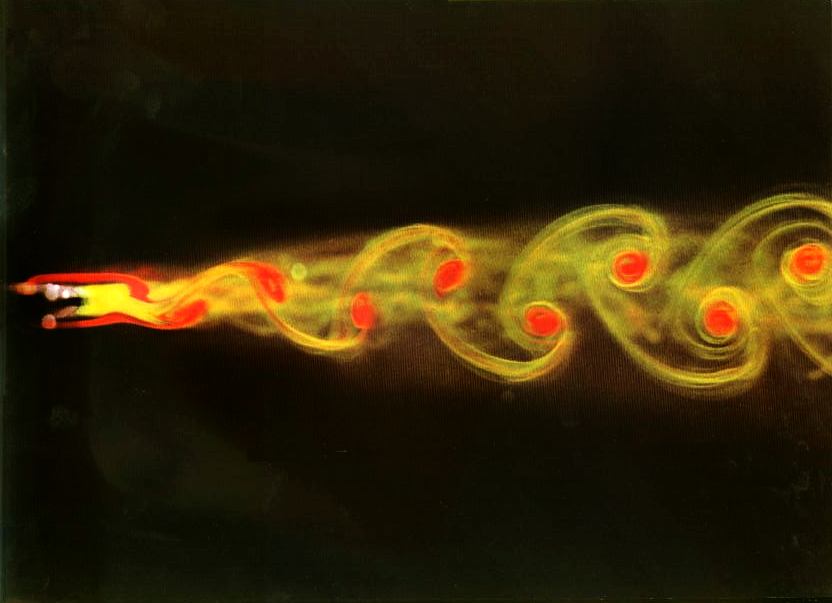
These things are not alive - in the sense in which we think about viruses and bacteria. But Jeremy England, a physicist from MIT, believes that the same physical principles can also be responsible for the emergence of life.

(Here you will find the full version of the work , which is very well readable, and here - slides to it ).
All reasoning is based on energy preferences — on what physicists call thermodynamics. The idea is that if you take a bunch of anything — atoms, molecules, etc. — and transfer energy to them in small and variable portions, they self-organize into more bound states. By the same principle, light and uneven shaking of a jar with nuts will cause the large nuts to rise, and the smaller ones to come down — this will be a more energy-stable configuration.
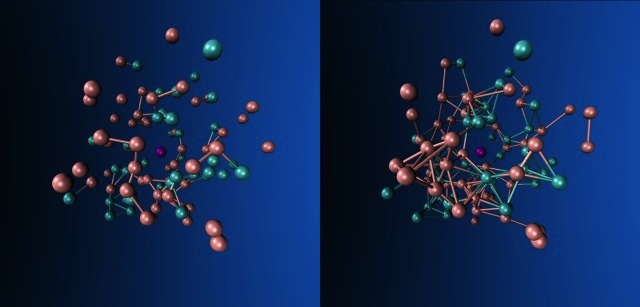
In terms of physics, England is right. And, nevertheless, most likely, this is not the whole history of the origin of life. There are two examples showing that evolution does not always (or even very often) find the most energetically beneficial solution to the problem.
Evolution did not lead to the appearance of wheels, although it would be energetically more efficient for a number of tasks.
And all plants at all times based the extraction of solar energy on two molecules: chlorophyll a and chlorophyll b. These are not the only molecules that can store solar energy or produce photosynthesis. Some bacteria use other molecules for this. Moreover, the spectrum of the sun is highest in the yellow / green range, while these molecules absorb the blue and red, but reflect the yellow / green.

But just because this phenomenon does not control everything in the world does not mean that it is not the cause of the emergence of life. Maybe it is. But at the moment, it's more like fantasy. As Harvard professor Yevgeny Shakhnovich said: “Jeremy’s ideas are interesting and promising, but at the moment extremely speculative, especially when applied to the phenomenon of life.”
Thermodynamics has many applications in biology and biological processes, but has life appeared according to the laws of entropy and thermodynamic processes?
This is possible, but agreement on this matter has not yet been reached, and will not be reached for a very long time. This hypothesis is very difficult to test, and we will not be sure of receiving confirmation until we have the holy grail of abiogenesis in our hands: until we create life from lifeless. We still do not know why and how life arose, but it is possible and reasonable to assume that thermodynamic processes will be able to answer this question. It is also great that people have long been engaged in the study of these issues, and that these studies lead us along such different and unexpected ways, some of which can lead us to answer questions about the origin and evolution of life on Earth.
Source: https://habr.com/ru/post/367835/
All Articles