American scientists have learned how to get alcohol with 99% purity using light
Experts from Rice University have developed a method of "optical distillation" of alcohol. According to scientists, led by Naomi Halas (Naomi Halas), this method requires much less energy than in the case of thermal distillation.
The authors of the methodology claim that the last few thousand years have heated the water incorrectly , starting from the bottom of the vessel. In fact, water heating should be started from above - this method is the most energy-efficient, since it does not require heating the entire volume of liquid.
Nanoparticles, which both absorb and scatter light when placed in a liquid, absorb optical energy and heat a certain volume of liquid due to scattering and optical absorption. This can provoke a local transfer of liquid to vapor within a certain volume, without the need to heat the entire liquid. For binary liquid mixtures, this process leads to the evaporation of the more volatile element of the mixture. If the volatile element is precipitated, this allows you to create a distillation process using nanoparticles, controlled by optical illumination. Since this does not require heating large volumes of liquid, the process requires significantly less energy than traditional distillation using heat sources and heating the liquid throughout the volume.
')
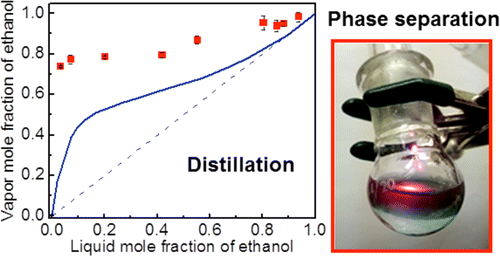
Scientists studied the behavior of illuminated ethanol (ethanol + water) and propanol (propanol-1 + water) mixtures using Au – SiO2 as nanoparticles. For ethanol mixtures, the molar fraction of ethanol obtained by exposing the mixture to optical radiation is substantially higher than in the case of thermal distillation.
The degree of distillation when using the optical method is much higher than thermal. In the first case, the distillate (alcohol) can be easily brought to a purity of 99% , in the second - only 95%.
The results of the work have already been published in the journal ACS Nano.
Source: https://habr.com/ru/post/367157/
All Articles