Stanford Improved Clean Way to Store Renewable Energy
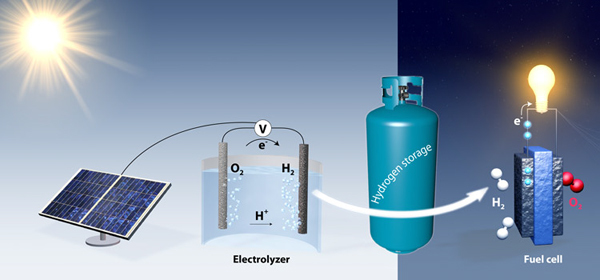 Researchers at Stanford University have found a catalyst for the production of large volumes of pure hydrogen by electrolysis of water, the process of passing electric current through the solution.
Researchers at Stanford University have found a catalyst for the production of large volumes of pure hydrogen by electrolysis of water, the process of passing electric current through the solution.Today, hydrogen H 2 is most often produced from methane CH 4 and other hydrocarbons. Tens of millions of tons of this most common gas in the Universe are produced every year; it is used in the chemical industry.
Professor of Chemical Engineering Thomas Jaramillo and his researcher Jacob Kibsgaard want to use the electrolysis process to extract hydrogen from water and use the resulting product for storing solar energy, but for the ubiquitous distribution of this approach they need to significantly reduce the cost of the process.
')
The process of electrolysis of water is as follows: two electrodes are lowered into the water, then electric current is passed through it, and bubbles of water-forming elements protrude on their surface. But this process requires a catalyst on the electrodes, and traditionally it is platinum, a rather expensive solution.
It is the replacement of this rare metal with something more affordable that is needed to reduce the cost of the electrolysis process. In the German scientific journal Angewandte Chemie, Jaramillo and Kibsgaard described a clean, reliable and effective substitute for precious metal. And their intentions go far beyond the current market of the chemical industry.
Now the use of solar panels largely rests on the storage and conversion of energy, common and cheap systems which are quite difficult to find. Researchers from Stanford believe that they are able to turn water tanks into batteries.
During the day, solar panels will produce electricity, and its surplus will be used to generate hydrogen and oxygen. At night, these gases are again connected to the water in the fuel cells, generating electricity for consumers of the electrical network. Unlike batteries, which include an electrolyte in its composition, potentially carrying great harm to the environment when it is not properly disposed of, such storage is clean and relatively safe.
At the nanoscale level, platinum in the electrolysis process fixes a bare proton of an atom of hydrogen that has lost its electron until the hydrogen atom can pick up an electron to form an H 2 molecule paired with another such hydrogen atom.
And the snag is to find a catalyst with a certain bond strength: if it is too weak, the hydrogen atom will not hold, if it is too strong, then it will not be released.
Platinum is ideally suited for this purpose, but last year at Stanford it was discovered that a variant of molybdenum sulfide, a catalyst widely used in oil refining for cracking heavy hydrocarbons, could be its counterpart. It turned out that some of its properties fit well.
In addition to this catalyst, researchers also borrowed another trick from the oil refining industry. For environmental reasons, sulfur is often removed from the oil during treatment to reduce the frequency of acid rain. Some sulfur atoms linger on the surface of the catalyst and increase its activity.
Already known for its positive effect on the electrolysis reaction of water, molybdenum phosphide was combined with sulfur. As a result, a new catalyst, molybdenum phosphosulfide, was obtained, which proved to be even more effective in the hydrogen evolution reaction than the original substance.
The new catalyst in terms of efficiency and durability is approaching platinum, which is very important in industrial installations where electrodes have to work for a long time. We are working on improving it at the nanoscale level.
Based on Stanford News .
Source: https://habr.com/ru/post/362823/
All Articles