Liquid breathing

This is probably a cliche in science fiction: a viscous substance enters the costume or capsule very quickly, and the main character suddenly discovers for himself how quickly he loses air remnants from his own lungs, and his insides are filled with an unusual shade fluid from lymph to blood . In the end, he even panics, but takes a few instinctual sips or, rather, sighs and discovers with surprise - he can breathe this exotic mixture as if he breathes ordinary air.
Are we far from the implementation of the idea of fluid breathing? Is it possible to breathe a liquid mixture, and is there a real need for this?
There are three promising ways to use this technology: it is medicine, diving to great depths and astronautics.
')
The pressure on the body of the diver increases with every ten meters per atmosphere. Due to a sharp decrease in pressure, a decompression sickness can begin, with manifestations of which the gases dissolved in the blood begin to boil with bubbles. Also, at high pressure, oxygen and narcotic nitrogen poisoning are possible. All these are struggling with the use of special breathing mixes, but they also do not give any guarantees, but only reduce the likelihood of unpleasant consequences. Of course, you can use diving suits, which maintain pressure on the body of the diver and his breathing mixture in exactly one atmosphere, but they, in turn, are large, bulky, difficult to move, and also very expensive.
Liquid breathing could provide a third solution to this problem with maintaining the mobility of elastic diving suits and the low risks of hard suits. In contrast to expensive breathing mixtures, respiratory fluid does not saturate the body with helium or nitrogen, therefore, there is also no need for slow decompression to avoid decompression sickness.
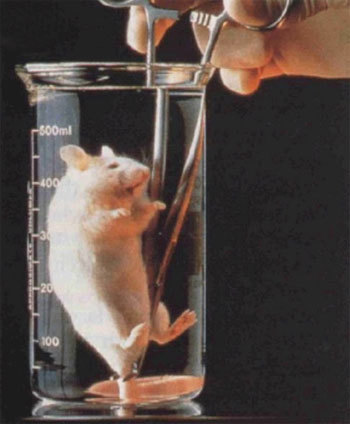 In medicine, liquid respiration can be used in the treatment of premature babies in order to avoid damage to the underdeveloped bronchi of the lungs by pressure, volume and concentration of oxygen of the air ventilators. Select and try different mixtures to ensure the survival of a premature fetus began in the 90s. It is possible to use a liquid mixture during full stops or partial respiratory failure.
In medicine, liquid respiration can be used in the treatment of premature babies in order to avoid damage to the underdeveloped bronchi of the lungs by pressure, volume and concentration of oxygen of the air ventilators. Select and try different mixtures to ensure the survival of a premature fetus began in the 90s. It is possible to use a liquid mixture during full stops or partial respiratory failure.Space flight is associated with large overloads, and liquids distribute pressure evenly. If a person is immersed in a liquid, then in case of overload the pressure will go on his entire body, and not on specific supports (backrests, seat belts). This principle was used to create a suit for Libelle overloads, which is a hard spacesuit filled with water, which allows the pilot to maintain consciousness and performance even with overloads above 10 g.
This method is limited by the difference in the density of tissues of the human body and the liquid used for immersion, so the limit is 15-20 g. But you can go ahead and fill your lungs with a liquid that is close in density to water. A cosmonaut completely immersed in a liquid and breathing with a liquid will feel relatively weakly the effect of extremely high overloads, since the forces in the liquid are distributed evenly in all directions, but the effect will still be due to the different density of the tissues of his body. The limit will still remain, but it will be high.
The first experiments on liquid breathing were carried out in the 60s of the last century in laboratory mice and rats, which were forced to inhale a saline solution with a high content of dissolved oxygen. This primitive mixture allowed animals to survive a certain amount of time, but it could not remove carbon dioxide, so irreparable harm was done to animals' lungs.
Later, work began on perfluorocarbons, and their first results were much better than the results of experiments with brine. Perfluorocarbons are organic substances in which all hydrogen atoms are replaced by fluorine atoms. Perfluorocarbon compounds have the ability to dissolve both oxygen and carbon dioxide, they are very inert, colorless, transparent, cannot damage lung tissue, and are not absorbed by the body.
Since then, breathing fluids have been improved, the most perfect solution at the moment is called perlublubron or “Liquive” (commercial name). This oil-like clear liquid with a density twice as high as water has many useful qualities: it can carry twice the amount of oxygen than ordinary air, has a low boiling point, so after use its final removal from the lungs is produced by evaporation. Alveoli under the influence of this fluid open better, and the substance gets access to their contents, it improves the exchange of gases.
Lungs can be filled with liquid completely, it will require a membrane oxygenator, a heating element and forced ventilation. But in clinical practice, they most often do not do this, but use liquid breathing in combination with ordinary gas ventilation, filling the lungs with perflubron only partially, approximately 40% of the total volume.
Shot from movie The Abyss, 1989
What prevents us from using liquid breathing? Breathing fluid is sticky and poorly removes carbon dioxide, so you will need forced ventilation of the lungs. To remove carbon dioxide from an ordinary person weighing 70 kilograms will require a flow of 5 liters per minute and above, and this is very much in view of the high viscosity of liquids. During physical exertion, the value of the required flow will only grow, and it is unlikely that a person will be able to move 10 liters of fluid per minute. Our lungs are simply not created for breathing with liquid and cannot pump such volumes by themselves.
The use of positive breathing traits in aviation and astronautics can also remain a dream forever - the fluid in the lungs for an overload protection suit should have water density, and a perflubron twice its weight.
Yes, our lungs are technically capable of “breathing” with a certain oxygen-rich mixture, but, unfortunately, we can only do this for a few minutes, because our lungs are not strong enough to circulate the breathing mixture for long periods of time. The situation may change in the future, it remains only to draw our hopes on researchers in this field.
According to zidbits.com and en.wikipedia.org .
Source: https://habr.com/ru/post/362705/
All Articles