What is the HL7 Continuity of Care Document (CCD)
As defined in a wiki, Continuity of Care Document or CCD, this is an XML-based standard aimed at coding the structure and semantics of a medical patient record for later exchange.
From the point of view of the developer of medical standards, CCD is a joint brainchild of the committees of HL7 International and ASTM (American Society for Testing and Materials). From a semantic point of view, CCD is the developer guide for implementing the ASTM CCR (Continuity of Care Record) standard based on CDA R2 (HL7 Version 3 Clinical Document Architecture Release 2). Here is such a tangled story.
Simply put, two committees met for a long time butting about standards and decided that all the data used in ASTM CCR (also known as ASTM E2369-05) would be encoded, with a few additions, in the CDA standard. What happened was called the Continuity of Care Document.
')
The standard is described in the following two documents available on HL7.org:
In addition to the developer’s manual itself, the standard includes CCD and HL7 core XML schemas, CCD CSS styles, and non-normative examples of Schematron rules for document validation.
Before proceeding further, a small digression, as in the general case, standards are developed HL7 and their artifacts. In HL7, this happens as follows, using the CCD as an example:
Graphically, this can be represented as follows (in this example, my own localization for generating XML schemas and all the required descriptions separately for the header and body of a CCD document):

Further, other organizations or business requirements may impose additional restrictions on the standard, for example, this happens in the USA with their Meaningful Use (MU) Stage 1 and Stage 2.
Templates
“Since the CDA specification is too flexible and multifunctional” (quoted from the Normative Edition HL7 in my free translation), the next level of restrictions for the CDA standard is called templates or patterns, which are defined at the level of documents, sections and records. The templates "must follow the detailed developer's guide, containing a detailed description of how the elements of the document should be structured and presented to ensure clinical safety." Again, the initial flexibility of the standard allows you to create many different templates.
So, at the moment, nine document templates are defined at the document level. In addition to CCD, there are Consultation Note, Diagnostic Imaging Report, Procedure Note, Discharge Summary, etc. There are about 65 templates defined at the section level and their number is likely to increase. Examples of sectional patterns are Allergies, Encounters, Medications, Problem List, etc.
The following picture schematically shows how document templates and section templates are grouped, as well as how additional restrictions are imposed, for example, Meaningful Use (MU).
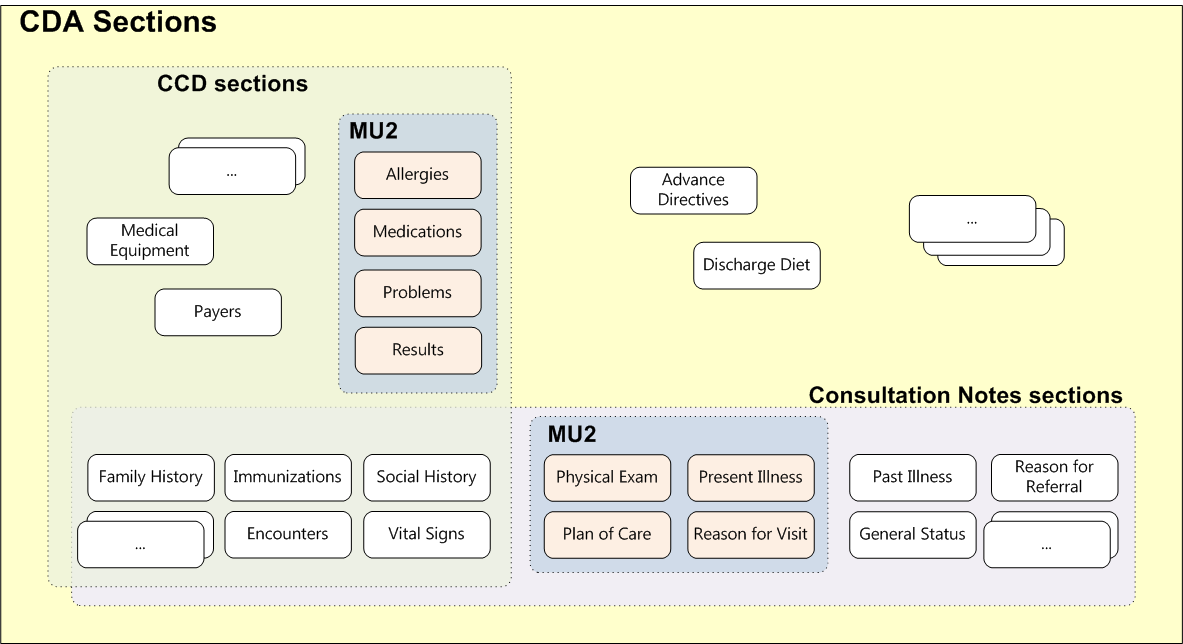
So, some section templates can be used as exclusively by some document templates, and included in several document templates. Naturally, the picture shows only a small part of the document templates and sections.
As mentioned above, CDA does not stop at sections and defines about a hundred record templates. Such patterns include, for example, Age, Observation, Encounter Diagnosis, Immunization Activity, etc. Record templates characterize one of the clinical conditions and imply only computer processing (that is, they are not intended to be read by human information). As with sections, some record templates are used exclusively in one type of document, while others may occur in several document types. For example, the Discharge Diet entry template is used only in the Discharge Summary document. And the template record is Reason for Referral only in the Referral Summary (also known as HITSP C48). In contrast, Allergies or Diagnostic Results records are found in all types of documents.
All additional information on CDA, CCD or HL7 is available, first of all, on the website HL7.org.
From the point of view of the developer of medical standards, CCD is a joint brainchild of the committees of HL7 International and ASTM (American Society for Testing and Materials). From a semantic point of view, CCD is the developer guide for implementing the ASTM CCR (Continuity of Care Record) standard based on CDA R2 (HL7 Version 3 Clinical Document Architecture Release 2). Here is such a tangled story.
Simply put, two committees met for a long time butting about standards and decided that all the data used in ASTM CCR (also known as ASTM E2369-05) would be encoded, with a few additions, in the CDA standard. What happened was called the Continuity of Care Document.
')
The standard is described in the following two documents available on HL7.org:
- HL7v3 Normative Edition - HL7 Clinical Document Architecture, Release 2.0;
- HL7 Implementation Guide: CDA Release 2 - Continuity of Care Document (CCD).
In addition to the developer’s manual itself, the standard includes CCD and HL7 core XML schemas, CCD CSS styles, and non-normative examples of Schematron rules for document validation.
Before proceeding further, a small digression, as in the general case, standards are developed HL7 and their artifacts. In HL7, this happens as follows, using the CCD as an example:
- The CDA document inherits classes from the HL7 RIM (Reference Information Model) in close collaboration with the HL7v3 data types.
- Further, the CCD document inherits its classes from CDA R-MIM (Refined Message Information Models).
- Further, on the CCD classes impose additional restrictions required by ASTM CCR.
- Further, based on the CCD R-MIM, all artifacts required by the HL7 standard are generated, such as XML schemas, MIF files and HMD (Hierarchical Message Descriptor) representations.
- In the final step, the CCD XML schema is manually adjusted to include the requirements originally missing from the HL7 RIM, but required by the ASTM CCR standard.
Graphically, this can be represented as follows (in this example, my own localization for generating XML schemas and all the required descriptions separately for the header and body of a CCD document):

Further, other organizations or business requirements may impose additional restrictions on the standard, for example, this happens in the USA with their Meaningful Use (MU) Stage 1 and Stage 2.
Templates
“Since the CDA specification is too flexible and multifunctional” (quoted from the Normative Edition HL7 in my free translation), the next level of restrictions for the CDA standard is called templates or patterns, which are defined at the level of documents, sections and records. The templates "must follow the detailed developer's guide, containing a detailed description of how the elements of the document should be structured and presented to ensure clinical safety." Again, the initial flexibility of the standard allows you to create many different templates.
So, at the moment, nine document templates are defined at the document level. In addition to CCD, there are Consultation Note, Diagnostic Imaging Report, Procedure Note, Discharge Summary, etc. There are about 65 templates defined at the section level and their number is likely to increase. Examples of sectional patterns are Allergies, Encounters, Medications, Problem List, etc.
The following picture schematically shows how document templates and section templates are grouped, as well as how additional restrictions are imposed, for example, Meaningful Use (MU).

So, some section templates can be used as exclusively by some document templates, and included in several document templates. Naturally, the picture shows only a small part of the document templates and sections.
As mentioned above, CDA does not stop at sections and defines about a hundred record templates. Such patterns include, for example, Age, Observation, Encounter Diagnosis, Immunization Activity, etc. Record templates characterize one of the clinical conditions and imply only computer processing (that is, they are not intended to be read by human information). As with sections, some record templates are used exclusively in one type of document, while others may occur in several document types. For example, the Discharge Diet entry template is used only in the Discharge Summary document. And the template record is Reason for Referral only in the Referral Summary (also known as HITSP C48). In contrast, Allergies or Diagnostic Results records are found in all types of documents.
All additional information on CDA, CCD or HL7 is available, first of all, on the website HL7.org.
Source: https://habr.com/ru/post/256021/
All Articles