Little things thinking or article about dendritic spines
A few months ago a series of articles was published under the general title “The Logic of Thinking” . Optimistically planned to continue it in a month or two. But life has made its own adjustments. Modeling the pattern-wave model of the cortex gave so interesting results that it was necessary to postpone for a while everything else, including writing the continuation of the cycle for Habr.
However, not so long ago I wrote and posted an article on the preprint ( http://arxiv.org/abs/1406.6901 ). In some ways, it may be of interest to those who have previously become interested in the wave model. Let me remind you that the key point of the model is the statement that neurons are able to memorize and recognize more than one single image described by the weights of its synapses, but also a huge number of other signals that are different from this image. Of course, this complication of the neuron goes against the many existing theories and requires more than a serious justification. Below, I will just try to describe one of the arguments given in the article in favor of my model.
This article should not be taken as a continuation of the cycle, it is rather a prequel to it. For myself, I called it the reasoning about the key role of dendritic spines.
')
Let's start by repeating the well-known.
In the state of rest between the internal and external environment of the neuron there is a potential difference - the membrane potential of about 70 millivolts. It is formed by protein molecules that work as ion pumps. As a result, the membrane acquires a polarization, in which a negative charge accumulates inside the cell, and a positive one outside.
The surface of the neuron is covered with branching processes - dendrites. Axonal terminations of other neurons adjoin the body of the neuron and its dendrites. The places of their connections are called synapses. Through synaptic interaction, a neuron is able to respond to incoming signals and, under certain circumstances, generate its own impulse, called a spike.
Signal transmission in synapses occurs due to the release of neurotransmitters. When the nerve impulse along an axon enters the presynaptic terminal, it releases from the synaptic vesicles the molecules of neurotransmitters characteristic of this synapse. On the membrane of a neuron that receives a signal, there are receptors that interact with neurotransmitters.
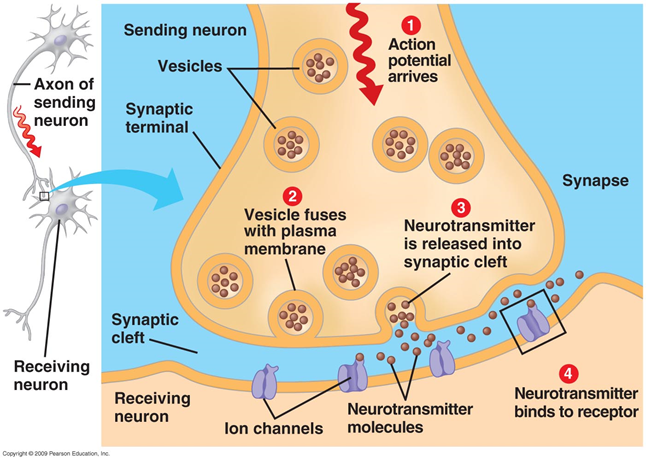
Figure 1. Chemical synapse
Receptors located in the synaptic cleft, for the most part are ionotropic. That is, they are also ion channels capable of moving ions across the neuron membrane. Neurotransmitters affect receptors in such a way that their ion channels open. As a result, the membrane is either depolarized or hyperpolarized, depending on which channels are affected and, accordingly, what type of synapse it is. In the excitatory synapses, channels open up, mainly transmitting cations into the cell, - the membrane depolarizes. In the inhibitory synapses, channels are opened, removing cations from the cell, which leads to hyperpolarization of the membrane.
In certain circumstances, synapses can change their sensitivity, which is called synaptic plasticity. This leads to the fact that some synapses become more, while others are less susceptible to external signals.
At the same time, a multitude of signals arrive at the neuron synapses. Braking synapses shift the potential of the membrane towards the accumulation of charge inside the cage. Activating synapses, on the contrary, try to defuse a neuron. When total depolarization exceeds the initiation threshold, a discharge occurs, called an action potential or a spike.
After the release of neurotransmitters, special mechanisms ensure their utilization and reuptake, which leads to clearing of the synaptic cleft and the space surrounding the synapse. During the refractory period coming after the spike, the neuron is not able to generate new impulses. The duration of this period determines the maximum generation frequency that a neuron is capable of.
Now we describe the facts less known.
When the action potential, extending along the axon, reaches the recipient neuron, it causes the release of neurotransmitters into the synaptic cleft. These mediators determine the synapse contribution to the total change in the membrane potential of a neuron receiving a signal. But part of the mediators falls outside the synaptic cleft and spreads over the space formed by the neurons and the glial cells surrounding them. This phenomenon is called spillover (spillover (eng.) - overflow, overflow) (Kullmann, 2000). In addition, mediators are emitted by non-synaptic axon terminals and glial cells (Figure 2). The concentration of neurotransmitters outside the synapses is much less than in the synaptic clefts. However, it is in these “spreading” neurotransmitters that a lot of interesting things lurk.
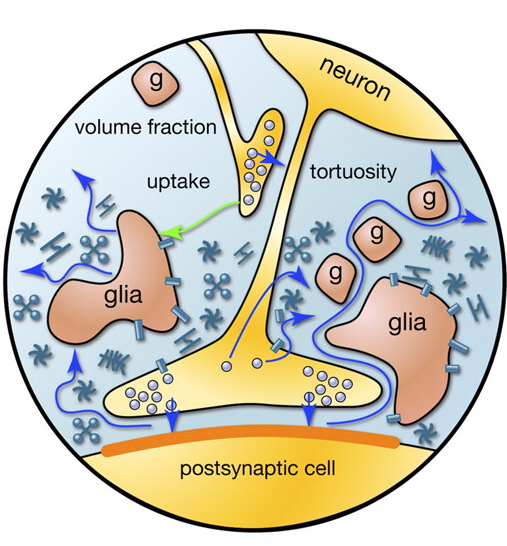
Figure 2. Sources of mediators outside the synaptic cleft (Sykova E., Mazel T., Vagrova L., Vorisek I., Prokopova-Kubinova S., 2000)
Let us try to estimate the number and structure of sources emitting neurotransmitters beyond the limits of synapses. To do this, we use the quantitative estimates of bark parameters given in the table below (Table 1) (Braitenberg V., Schuz A., 1998).
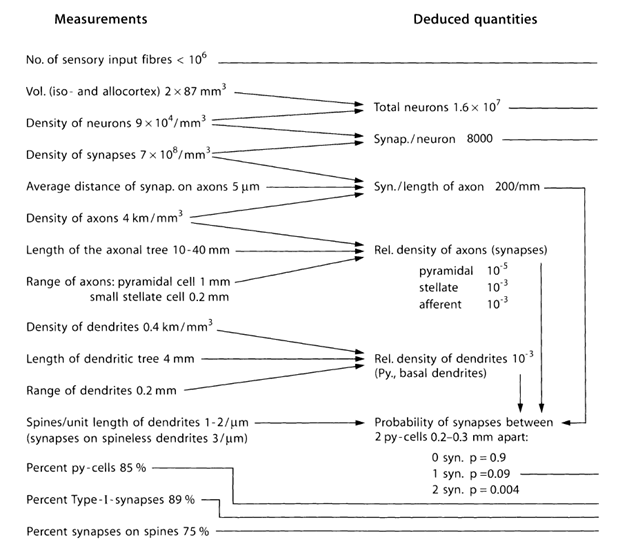
Table 1. Summary table of parameters obtained in the study of the mouse brain (py cell - pyramidal cell, Type-I - synapses between two pyramidal cells) (Braitenberg V., Schuz A., 1998)
Let me remind you that most of the synapses (90-95%) fall not on the body of the neuron, but on its dendrites. Dendrites are thin branching processes that form the so-called dendritic neuron tree. In the pictures below, the dendritic trees are highlighted in black and the axons in gray. For neurons of different types, the forms of dendritic trees are different, but the general principle is preserved: a dendritic tree consists of a set of branching processes, while the highest density of synaptic connections of a neuron falls on a small spatial region. For the main types of neurons, it is about 200 µm (Figure 3, Figure 4).

Figure 3. The structure of a star neuron, ruler - 0.1 mm (Braitenberg, 1978)
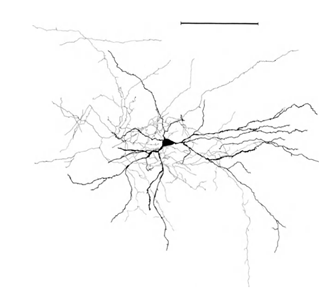
Figure 4. The structure of the pyramidal neuron, ruler - 0.1 mm (Braitenberg, 1978)
Branching axons of neurons form contacts (synapses) with the dendrites of other neurons. The average distance between synapses on dendrites is 0.5 micrometers. The average distance between synapses on axons is 5 micrometers, that is, 10 times larger. Not surprisingly, axons are about 10 times longer than dendrites.
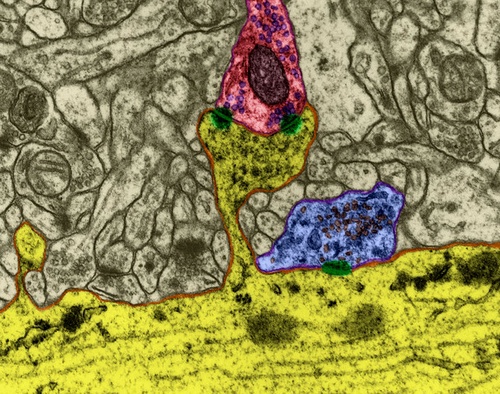
Most (75%) of the synapses are located on dendritic spines, which is most characteristic of pyramidal cells (Figure 5).
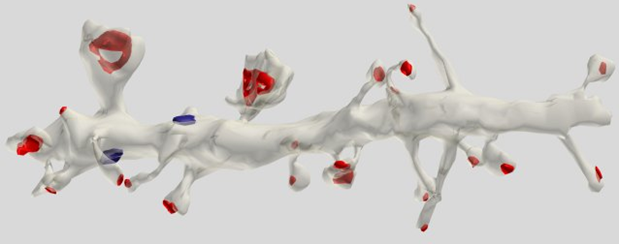
Figure 5. A segment of a pyramidal dendrite cell. Synapses on spines are marked in red, on dendritic stem in blue (Dr. Kristen M. Harris)
Computer simulations based on real anatomical and physiological data have shown that, for example, glutamate can spread beyond the synaptic cleft in amounts sufficient to activate NMDA receptors in a radius comparable to the distance between adjacent synapses (0.5 µm) (Rusakov DA, Kullmann DM, 1998). It can be assumed that a significant concentration of neurotransmitters after spillover is observed on a dendrite site of about 1-2 μm in length. On such a site can be located about two to four synapses belonging to this dendrite.
If you take a dendrite section with a length of 5 μm (Figure 6), then the expected number of synapses on it will be about 10.
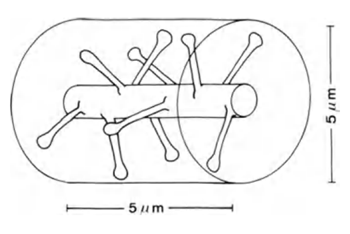
Figure 6. Plot of dendrite (Braitenberg V., Schuz A., 1998)
But the dendritic branches of some neurons are closely intertwined with the branches of other neurons. They pass from each other in the immediate vicinity. Due to the height of dendritic spines, synapses belonging to one dendrite may be closer to the surface of another dendrite than its own synapses.
If the synapses were evenly distributed in the space of the cortex, then about 100 synapses would fall into a cylindrical volume with a height of 5 μm and a diameter of 5 μm (figure above) with a synapse density of 7x10 8 / mm 3 . That is, 10 times more than what is directly located on the branch itself. In reality, glial cells and bodies of neurons occupy a substantial part of the brain volume, which further increases the packing density of synapses. However, neurons work with different neurotransmitters, which must also be taken into account.
Now let us try to understand the meaning of such a structure of relations from the point of view of the distribution of the density of the extrasynaptic mediator. To do this, we use the simplified model. Take the conditional volume surrounding the neuron and number the neurons it contains. Each of these neurons will have:
• several synaptic contacts with the dendrite of the selected neuron;
• several “adjoining”, that is, places where its synapses with other neurons will be located in the immediate vicinity of the dendrite of the selected neuron.
Imagine a dendritic tree as one long branch with uniformly distributed conditional sources (Figure 7). For each source on this branch, you can specify the number of the neuron from the surrounding space, responsible for it. Each of the neurons of the environment will have several source contacts at once, randomly distributed over the dendrite. We denote this correlation by the vector D with the elements d i .
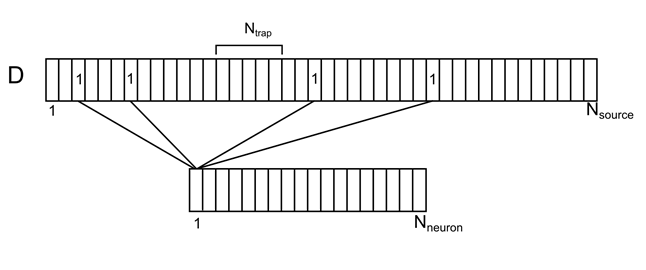
Figure 7. Correlation of surrounding neurons and their contacts on the dendrite
We introduce the notation:
N neuron - the number of neurons of the environment
N source - the number of sources for one neuron
N trap - the number of sources that create the level of density of neurotransmitters (synaptic trap)
Now suppose that several neurons from the environment gave a spike. This can be perceived as a signal available to observe our neuron. Let N sig - the number of active neurons that create the information signal. We write this signal with a binary vector S.
For all positions on the dendrite except the most extreme ones, we will assume the density of the mediator by the formula
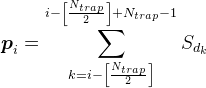
For example, for the signal shown in the figure below, the density in the marked synaptic trap will be 2 (the sum of the signals from the 1st and 4th neurons).
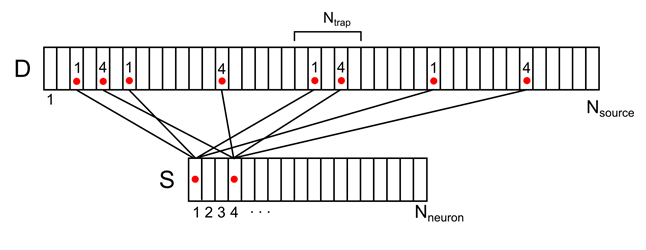
Figure 8. Mapping the activity of two neurons of the environment to the dendritic tree (only part of the connections and numbering is shown)
For any arbitrary signal, it is possible to calculate the pattern of density of mediators on the dendrite. This density will range from 0 to N trap . The maximum value will be achieved if all the sources that form the synaptic trap were active.
We use the averaged values of the parameters characteristic of the real rat cortex (Braitenberg V., Schuz A., 1998). Based on them, we get the following model parameters:
N neuron = 650
N source = 25000
N trap = 15
We will assume that the signal is encoded by activity, for example, 1.5% of cortical neurons, then
N sig = 10
It is easy to calculate the probability that for an arbitrary signal consisting of N sig units, there is at least one place on the dendrite, where the density of the mediator is exactly K. For the above parameters, the probability takes the following values (Table 2):
| K | P |
| 0 | 0.984 |
| one | one |
| 2 | one |
| 3 | 0.996 |
| four | 0.287 |
| five | 0.016 |
| 6 | 0.001 |
| 7 | 0 |
| eight | 0 |
| 9 | 0 |
| ten | 0 |
| ... |
Table 2. Table of the probability of finding at least one trap with a given density. The first column is the required number of active sources in the trap. The second is the probability of finding at least one place on the dendrite, where there will be just such a number of active sources.
That is, for those values of the parameters that are close to the configuration of the real cortex, for any volume signal affecting about 1.5% of neurons, the following is true:
• There are about 1.6% of neurons in which there is a trap on dendrites, where 50% of signaling axons intersect;
• Practically every neuron has traps in which at least 30% of its signaling axons intersect.
The meaning of this result is very interesting. Suppose that the information in the cortex is somehow encoded by synchronous activity of a relatively small number (N sig ) of compactly located neurons. This is not about all brain activity, but about information processes in a small volume, where we number the neurons from 1 to N neuron . Suppose that the number of code combinations S is limited and forms a certain dictionary T with capacity N dict . You can calculate the probability that the same place will "respond" to two signals at once. The results of this calculation for a dictionary of 10,000 signals in the table below.
| K | P error |
| 3 | 0,00399 |
| four | 1,05E-05 |
| five | 1.89E-08 |
| 6 | 2.33E-11 |
| 7 | 0 |
| ... |
Table 3. The table of the probability of violation of the synaptic trap uniqueness at different levels of the density of the mediator
It turns out that, at K = 3, the traps have a certain selectivity, although they do not guarantee against error, but already at K = 5, they begin to quite uniquely correspond to a certain spatial pattern of activity. Let me remind you that this is true not for arbitrary signals from an infinite set, but when we have a set, albeit a sufficiently large, discrete allowed states of activity.
That is, it turns out that the meaning of the axon and dendritic trees structure inherent in the brain is to create traps on the dendritic surface of each neuron, that is, places corresponding to various combinations of sources of neurotransmitters. The density of the neurotransmitter in the traps can be judged on the spatially distributed signal, which is the sum of the synchronous activity of a number of nearby neurons.
And since the so-called metabotropic receptors are located on the surface of the neuron, which can cause single neuron spikes even with a slight concentration of neurotransmitters, it turns out that these spikes, which are commonly called spontaneous, can neuron react to a huge number of different signals that differ from those neuron synapses can be configured.
Actually, it is now possible to explain the purpose of the very dendritic spines from which the article began. In theory, all the synapses could be located directly on the dendrite or the body of the neuron. This would have no effect on the direct work of the synapses and their ability to contribute to the appearance of the induced activity. But now we can assume that the appointment of dendritic spines is the creation of a spatial structure in which the synapses of different neurons are “mixed” in such a way that they acquire the ability of their spilover to influence not only their surface, but also the surrounding neighboring dendrites. Such a “trifle” is obtained.
The initial article itself shows how the “wave story” itself is born from all this, but this is a separate conversation.
And in the end about a little self-interest. Original article in Russian. There is a translation into English made by Dmitry Shabanov (for which he is very respectful), but he (the translation) is far from perfect. If anyone has the opportunity to look and point out mistakes, I will be extremely grateful. The document on the link is open for everyone to comment.
References to the original article
1. (2014). Derived from the Human Connectome Project: www.humanconnectomeproject.org
2. (2014). Obtained from ALLEN Mouse Brain Connectivity Atlas: connectivity.brain-map.org
3. Bloom, BH (1970). Space / time trade-offs in hash coding with allowable errors. Communications of the ACM T. 13 (7), 422–426.
4. Braitenberg V., Schuz A. (1998). Cortex: statistics and geometry of neuronal connectivity, 2nd ed.
5. Braitenberg, V. (1978). Cortical architectonics: general and areal. In MAB Brazier and H. Petsche (eds), Architectonics of the Cerebral Cortex (pp. 443–465). New York: Raven Press.
6. Coster, H. (1975). Electromechanical stresses and the effect of pH on membrane structure. Biochim Biophys Acta 13; 382 (2), 142-146.
7. Dr. Kristen M. Harris. (b.d.). Synapse Web. Obtained from synapses.clm.utexas.edu .
8. Fukushima, K. (1980). Neocognitron A self-organizing recognition of the pattern of recognition of the unaccepted by a shift in position. Biological Cybernetics, 36 (4), 193-202.
9. Grossberg, S. (1987). Competitive learning. Cognitive Science N11, 23-63.
10. Hebb, D. (1949). The Organization of Behavior. New York: John Wiley & Sons.
11. Hodgkin, A. a. (1952). A quantitative description of the current ... J. Physio l. 117, 500-544.
12. Izhikevich, EM (2007). Dynamic Systems in Neuroscience: The Geometry of Excitability and Bursting. London: The MIT Press.
13. Kullmann, DM (2000). Spillover and synaptic cross talk mediated by glutamate and GABA in the mammalian brain. Prog Brain Res, 125, 339-351.
14. Kuramoto, Y. (1984). Chemical Oscillations, Waves, and Turbulence. Dover Publications.
15. Liibke J., Markram H., Frotscher M., Sakmann B. (1996). It was established by layer 5 of the pyramidal neurons in comparisons with the synaptic innervation of the same class. Neurosci 16 (10), 3209-3218.
16. Malenka RC, Nicoll RA (1999). Long-term potentiation - a decade of progress? Science 285 (5435), 1870-1874.
17. Michael T. Lippert, Kentaroh Takagaki, Weifeng Xu, Xiaoying Huang, Jian-Young Wu. (2007). Methods for Voltage-Sensitive Dye Imaging of Rat Cortical Activity With. J Neurophysiol 98, 502-512.
18. Pitts, W., McCulloch WS (1947). Auditory and visual forms. Bull. Math Biophys V.9,127–147.
19. Rosenblatt, F. (1962). Principles of Neurodynamic: Perceptrons and the Theory of Brain Mechanisms.
20. Rusakov DA, Kullmann DM (1998). Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. Neurosci 18 (9), 3158-3170.
21. Sheng, M., Nakagawa, T. (2002). Neurobiology: glutamate receptors on the move. Nature, 417 (6889), 601-602.
22. Sheng, M., Sala C. (2001). PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci, 24, 1-29.
23. Sykova E., Mazel T., Vagrova L., Vorisek I., Prokopova-Kubinova S. (2000). Extracellular space diffusion and pathological states. Progress in Brain Research, 155-178.
24. Tovar KR, Westbrook GL (2002). Mobile NMDA receptors at hippocampal synapses. Neuron, 34 (2), 255-264.
25. W.-F. Xu, X.-Y. Huang, K. Takagaki, and J.-Y. Wu. (2007). Compression and reflection of visually evoked cortical waves. Neuron, 55, 119-129.
26. Y. LeCun, Y. Bengio. (1995). Convolutional Networks for Images, Speech, and Time-Series, in Arbib, MA, editor, The Handbook of Brain Theory and Neural Networks. MIT Press.
27. J.P. Shnurova, Z.M. Gvozdikova. (1971). The reaction of the neurons of the sensorimotor region of the cortex to its direct electrical stimulation. In the Collection "Study of the organization of the neural activity of the cerebral cortex" (p. 158-180). Moscow: Science.
28. Nicholls, J., Martin, R., Wallace, B., Fuchs, P. (2003). From Nero to the brain (fourth edition).
29. Pribram, K. (1971). Brain languages.
30. , . . (2007). . .
31. , . (2014). . aboutbrain.ru: www.aboutbrain.ru/programs
32. , . (2014). . aboutbrain.ru: www.aboutbrain.ru/programs
Source: https://habr.com/ru/post/230047/
All Articles