Power sources

Stagnation in battery design is obvious to all. Mobile devices, at best, stay on one charge for two days, and this is considered an excellent indicator. But most often our smartphones and tablets hardly live to see the evening. Over the past decade, the power consumption of mobile devices has grown many times, but batteries are treading water.
This was one of the reasons why we use YotaPhone electronic ink displays in both generations, they are extremely economical. But the problem of power sources is very important not only for smartphones and tablets. Many devices and gadgets use batteries and rechargeable batteries. And therefore we want to offer you as a material for reflection a translation of an article in which it is interesting and detailed about the past, present and possible future of wearable power sources.
Few people remember about electric batteries until the moment when they are discharged. But this utilitarian and unexpectedly ancient technology has given a lot to human civilization. In fact, the modern world would not exist in its current form if it were not for the batteries with their completely unique ability to store and deliver electrical energy. Without batteries, there would be no mobile electronics, any compact electrical devices, including flashlights. We would be tightly “tied” to stationary outlets. Let's remember together the history of the emergence of this technology, evaluate its current state and try to predict further development.
')
Historical excursion
Baghdad battery
 In 1936, in the ruins of a village in the outskirts of Baghdad, we found terracotta jugs covered with something strange. Their age was dated either by the era of the Parthian kingdom or the Sassanian empire . The purpose of the jars remained unknown, and they were given to the National Museum of Iraq. 2 years later, they caught the eye of the German archaeologist Wilhelm Koenig. After some time, he published a report in which he suggested that these jugs are the forerunner of a galvanic cell .
In 1936, in the ruins of a village in the outskirts of Baghdad, we found terracotta jugs covered with something strange. Their age was dated either by the era of the Parthian kingdom or the Sassanian empire . The purpose of the jars remained unknown, and they were given to the National Museum of Iraq. 2 years later, they caught the eye of the German archaeologist Wilhelm Koenig. After some time, he published a report in which he suggested that these jugs are the forerunner of a galvanic cell .The pitchers were 14 cm high, inside there was a small copper tube (rolled out of a sheet). A rusty iron bar was inserted into the tube using an asphalt plug. The jug could be filled with a solution of acid or alkali: lemon, grape juice or vinegar. Researchers believe that such a battery gave a weak but stable voltage from 0.8 to 2 V. It is also believed that these jars were used in religious or healing ceremonies. In ancient times, electric batteries were generally used as a means of treatment because they were a mysterious phenomenon, a mystery of life. But to this day, no analogues of the Baghdad battery have been found, so the medical version is not backed up with respect to it.
Galvanic cell
 As you know from the school biology course, frog legs are used to demonstrate muscle contraction under the action of an electric current. This phenomenon was discovered in 1771 by Luigi Galvani, a professor at the University of Bologna. According to the legend, he removed skin from a frog, stuck with copper pins to the table, on which he had previously conducted experiments with static electricity. Assistant Galvani took a scalpel from the table (which, by chance, was charged during a previous experiment) and touched the frog's bare nerve. There was a spark, the leg jerked, and Galvani dawned that the electric charge is transported by ions.
As you know from the school biology course, frog legs are used to demonstrate muscle contraction under the action of an electric current. This phenomenon was discovered in 1771 by Luigi Galvani, a professor at the University of Bologna. According to the legend, he removed skin from a frog, stuck with copper pins to the table, on which he had previously conducted experiments with static electricity. Assistant Galvani took a scalpel from the table (which, by chance, was charged during a previous experiment) and touched the frog's bare nerve. There was a spark, the leg jerked, and Galvani dawned that the electric charge is transported by ions.In fact, he did not think so literally. Galvani decided that “animal electricity”, which is born in tissues, is transmitted by “electric fluids”. This erroneous view will last in science for about 30 years. However, the discovery of the Galvani marked the beginning of modern batteries and was called the “galvanic cell”.
Volt pole
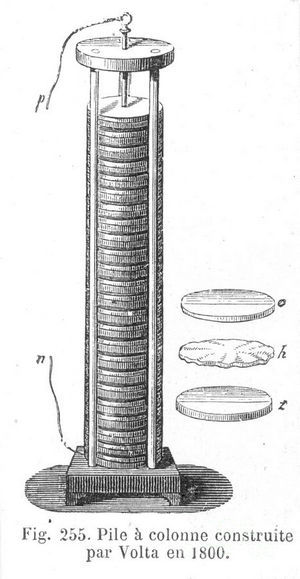 Galvani was of the opinion regarding the origin of electricity in living things until the end of life. He believed that it produced femoral muscles. One of the first contemporaries of Galvani, who reproduced his experience, was Alessandro Giuseppe Antonio Volta, a professor of experimental physics at the University of Pavia. Volta noticed that the frog's paws not only reacted to electricity, but also carried it out.
Galvani was of the opinion regarding the origin of electricity in living things until the end of life. He believed that it produced femoral muscles. One of the first contemporaries of Galvani, who reproduced his experience, was Alessandro Giuseppe Antonio Volta, a professor of experimental physics at the University of Pavia. Volta noticed that the frog's paws not only reacted to electricity, but also carried it out.To prove this, the professor assembled a setup consisting of a stack of silver and zinc discs separated by a brine-soaked cloth. Volta wanted to make this stack as high as possible. This is how the “volt pillar”, the world's first electric battery, was born. Thanks to the "pillar" Volta discovered the law of the name of itself about the potential difference.
"Voltaic pole" led to a number of discoveries made by other scientists, for example, the phenomenon of electrolysis. Various liquids were used as electrolytes of steel, including hydrochloric acid solution.
The most significant modification of the invention of Volta was created in 1866. Georges Leclanche placed a zinc anode and a cathode of manganese dioxide and carbon in a solution of ammonium chloride. As a result, a voltage of 1.4 V occurred (the original “pillar” allowed it to reach 0.4 V). This is equivalent to the voltage of modern alkaline batteries. Leclanche's invention was used in the first telephone systems and laid the foundation for modern dry cell batteries that are used in most gadgets.
Species diversity
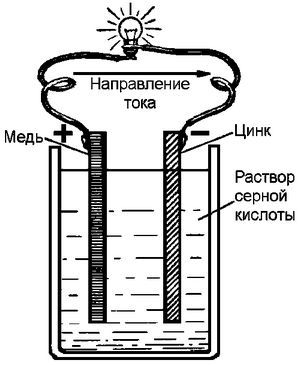 All batteries are divided into two classes: primary and secondary. Most primary batteries are alkaline with a zinc or lithium anode. They are quite cheap in production and are intended for disposal after the charge is exhausted.
All batteries are divided into two classes: primary and secondary. Most primary batteries are alkaline with a zinc or lithium anode. They are quite cheap in production and are intended for disposal after the charge is exhausted.Secondary batteries (accumulators) are mostly represented by three types: lead-acid, nickel, and lithium-ion batteries. In production, they are somewhat more expensive than the primary ones. They can be used repeatedly, which allows you to significantly save money and pollute the environment less.
Batteries are classified not only by the possibility of reuse, but also by the type of elements used:
• Galvanic (liquid) elements . The oldest type of battery that uses liquid electrolyte to "transport" ions. Widely used in the automotive industry and to power various home devices.
• Dry items . They are arranged almost the same as electroplating, except for the absence of liquid in their composition: the electrolyte is contained in the form of a wet paste, which is much safer. Can be used to create primary and secondary batteries.
• Elements with salt melt . Used in industry. In them, the salt is heated to the melting temperature and this liquid plays the role of an electrolyte. This solution allows to achieve high electric capacity of batteries.
• Reserve items . The electrolyte tank is separated from the cells, which allows them to be stored for a long time without losing charge. Such structures are most often used for scientific and military purposes.
What unites all types of batteries? The fact that they are all variations of a volt element that generates electricity due to a chemical reaction. Each element conventionally consists of three components: two electrodes and an electrolyte, through which the ions flow during the redox reaction.
With such a variety of types of batteries, they can be compared with each other, at first glance, by capacity. However, this is true only if the batteries are the same in size and design. Battery life depends significantly on its type and type of device in which it is used. For example, primary alkaline batteries usually have a higher maximum capacity, but in fact they reach it when the current consumption is rather weak: in smoke detectors, children's toys, etc. Lithium secondary batteries, on the other hand, usually have a slightly lower capacity, but work longer and are more stable in high-power devices, such as digital cameras. Let's take a closer look at each type of battery.
Primary
 Alkaline batteries are the most common, they occupy about 70% of the market. According to data for 2011, 10 billion pieces were produced worldwide. Most often in these batteries the anode is made of zinc powder, which increases the total surface area and contributes to the flow of current. The cathode is usually made from a mixture of manganese dioxide and carbon. Unlike other batteries with a zinc anode, potassium hydroxide is used as the electrolyte in the alkaline, and not ammonium or zinc chloride. Therefore, alkaline batteries have a higher specific capacity, they last and last longer (3-5 years, normal zinc - 2-3 years). Initially, the voltage is 1.55-1.7 V, gradually decreasing to 0.8. Low temperature has very little effect on performance.
Alkaline batteries are the most common, they occupy about 70% of the market. According to data for 2011, 10 billion pieces were produced worldwide. Most often in these batteries the anode is made of zinc powder, which increases the total surface area and contributes to the flow of current. The cathode is usually made from a mixture of manganese dioxide and carbon. Unlike other batteries with a zinc anode, potassium hydroxide is used as the electrolyte in the alkaline, and not ammonium or zinc chloride. Therefore, alkaline batteries have a higher specific capacity, they last and last longer (3-5 years, normal zinc - 2-3 years). Initially, the voltage is 1.55-1.7 V, gradually decreasing to 0.8. Low temperature has very little effect on performance.The cheapest in the production of primary elements - dry carbon-zinc. Often free of charge come bundled with the main product. It is most expedient to use them in devices with low and medium power consumption: remote controls, lamps, watches. Differ in low duration of work.
In zinc chloride elements, the specific capacity is about 50% higher than that of carbon-zinc elements. They are 20% heavier, they work much longer with high energy consumption, they are stored longer, they keep their charge better at low temperatures.
There is a separate type of alkaline batteries in which the anode is made of lithium or its alloys. The manganese dioxide cathode is immersed in an electrolytic paste of dissolved lithium salts. AA and AAA batteries can range in voltage from 1.5 to 3.7 V. Lithium alkaline batteries work about twice as long as alkaline under conditions of high power consumption, due to high capacity and low internal resistance. Shelf life reaches 10 years. But all this is reflected in their price.
What is the most efficient use of primary batteries? First, it’s best to keep them in the fridge. This allows you to slow down the chemical reaction occurring in the batteries, and extend the shelf life by about 5%. Do not forget to put them in a package without moisture, so as not to corrode. Allow time to warm to room temperature before use.
Some manufacturers, by contrast, recommend storing batteries at a temperature of 18-25 degrees Celsius and a relative humidity of 35-65%. Under these conditions, alkaline batteries are stored for 5-7 years, carbon-zinc - 3-5, lithium - 10-15 years.
Avoid storing primary batteries at temperatures above 25 degrees, this greatly accelerates their self-discharge. Never try to charge them, it will cause an explosion. A spray of hot electrolyte will bring you a little pleasure.
Secondary
This type of battery - batteries - it is possible to direct the chemical reaction in the opposite direction. To do this, you must forcibly change the direction of current flow. As already mentioned, there are three main types of batteries: lead-acid, nickel and lithium-ion. They are very widely used in many areas, from cell phones to computer centers.
<The first type of battery does not apply to electronics and gadgets, so we consider only two other types.>
 Nickel batteries are divided into nickel-cadmium (NiCd) and nickel metal hydride (NiMH). In the first electrodes are made of nickel hydroxide and cadmium. Nickel-cadmium batteries produce a voltage of about 1.2 V, work well at low temperatures, capacity and service life do not decrease with high power consumption. However, they have a rather short shelf life.
Nickel batteries are divided into nickel-cadmium (NiCd) and nickel metal hydride (NiMH). In the first electrodes are made of nickel hydroxide and cadmium. Nickel-cadmium batteries produce a voltage of about 1.2 V, work well at low temperatures, capacity and service life do not decrease with high power consumption. However, they have a rather short shelf life. Nickel-metal hydride batteries are much more common. The anode instead of cadmium is made of an alloy of rare-earth metals (lanthanum, cerium, neodymium and praseodymium). Potassium hydroxide is used as an electrolyte. At a voltage of 1.2 V, the capacity of NiMH batteries is 2-3 times higher than that of NiCd, and is comparable to lithium-ion batteries.
Nickel-metal hydride batteries are much more common. The anode instead of cadmium is made of an alloy of rare-earth metals (lanthanum, cerium, neodymium and praseodymium). Potassium hydroxide is used as an electrolyte. At a voltage of 1.2 V, the capacity of NiMH batteries is 2-3 times higher than that of NiCd, and is comparable to lithium-ion batteries.NiMH batteries are widely used in hybrid cars, and in general, NiCd has been almost completely superseded. Their duration of work with high power consumption is much longer than that of alkaline batteries. The main drawback is a very fast self-discharge, reaching 20% on the first day and about 4% each subsequent week. High temperature greatly reduces service life.
How to use NiMH batteries most effectively? With intensive charging mode, the internal resistance and the likelihood of overcharging increase, which shortens the service life and can lead to failure. If you need to store batteries for a long time, discharge it by about 60% and put it in the refrigerator. Some manufacturers recommend completely discharging the battery, and then shorting the contacts before long-term storage. It is also useful to do this in normal use about every 30 cycles to reduce the “memory effect”, which results in a gradual decrease in battery capacity.
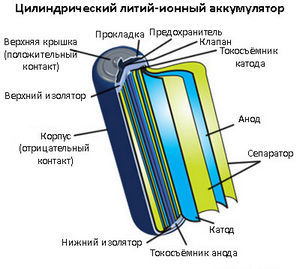
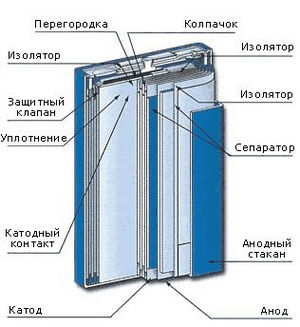 Lithium-ion (Li-ion) batteries are most often used in various electronics: smartphones, tablets, laptops, walkie-talkies, etc. The anode is most often a stack of millions of thin sheets of graphite. Compared to NiMH, Li-ion batteries are smaller, lighter, have a higher specific capacity, work well over a wide range of temperatures. They have a much lower self-discharge rate compared to other secondary batteries: 5-10% per month.
Lithium-ion (Li-ion) batteries are most often used in various electronics: smartphones, tablets, laptops, walkie-talkies, etc. The anode is most often a stack of millions of thin sheets of graphite. Compared to NiMH, Li-ion batteries are smaller, lighter, have a higher specific capacity, work well over a wide range of temperatures. They have a much lower self-discharge rate compared to other secondary batteries: 5-10% per month.They also have serious flaws. They are quite expensive to produce and recycle. Overcharging causes overheating, melting and, possibly, explosion, and a deep discharge - to short circuit. To avoid this, small control circuits are built into lithium-ion batteries. But it also reduces the charging speed. Prone to the "memory effect", therefore, come into disrepair after several years of operation or several hundred cycles.
 How to use Li-ion batteries most effectively? Under normal use, they serve 3-5 years. High temperature reduces this figure, it is better to use and store them at room temperature. Since the capacity gradually decreases with each discharge, it is better to periodically recharge the battery. This does not mean that you need to constantly keep it fully charged, on the contrary - all secondary batteries need periodic "rest."
How to use Li-ion batteries most effectively? Under normal use, they serve 3-5 years. High temperature reduces this figure, it is better to use and store them at room temperature. Since the capacity gradually decreases with each discharge, it is better to periodically recharge the battery. This does not mean that you need to constantly keep it fully charged, on the contrary - all secondary batteries need periodic "rest."Do not leave the electronics for a long time connected to the network, this leads to the oxidation of the cells and greatly reduces the service life. Before long storage, it is better to recharge the battery a little: many manufacturers recommend leaving no more than 40%.
Batteries of the future
Due to its relatively high specific capacity, lithium-ion batteries occupied a dominant position. However, the increase in power consumption of devices is outpacing the possibilities for increasing the efficiency of batteries. Therefore, alternative technologies are being actively explored, for example, lithium-silicone, in which the battery increases in volume by 3 times when fully charged and gradually “blown away” when exhausted. In this case, the capacity of such batteries can be 10 times higher than the capacity of Li-ion. Another candidate is lithium-air technology. Such batteries may have a very high energy density, comparable to an internal combustion engine. You can also mention the supercapacitor technology, which involves "storing energy using charge resonating on a large surface of the material in the design of the device."
Conclusion
The first commercial sample of lithium-ion batteries appeared in 1991. In 1996, an improved version appeared - a lithium-polymer battery. 18 years have passed since then, and nothing significant has been proposed. Compare how much the power consumption of devices increased during this period. It is difficult to say when scientists will be able to offer an adequate replacement for modern batteries. It has long been the need for a new technology, which, with comparable dimensions, will ensure the capacity of batteries at least 3-5 times higher than now. And there is still no encouraging news from the camp of developers.
It is believed that in the next decade a breakthrough in this area will not occur. So the creators of gadgets will have to rely only on the capabilities of current battery technology. Therefore, the use of E-ink display in YotaPhone remains appropriate. In any case, we are closely following the innovations in the field of power supplies.
We are also interested to know your opinion on the current situation in the field of compact power sources. Do you share the forecast for the next decade of stagnation? Maybe you have information about promising power sources, which may soon change existing technologies?
Source: https://habr.com/ru/post/228159/
All Articles