Like graphene, only from phosphorus
Scientists are actively exploring the possibility of obtaining new materials similar to graphene - consisting of a layer of a substance one atom thick. Substantial progress has recently been demonstrated in the production of phosphorus, a material consisting of a single layer of phosphorus atoms.
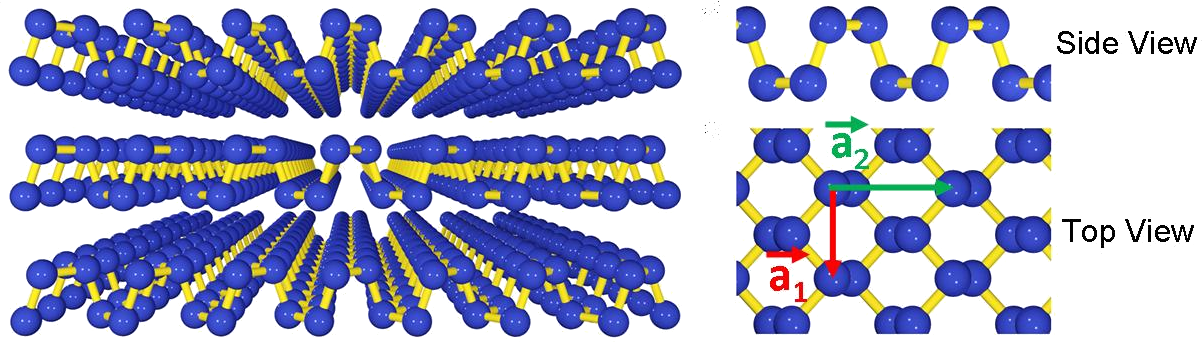
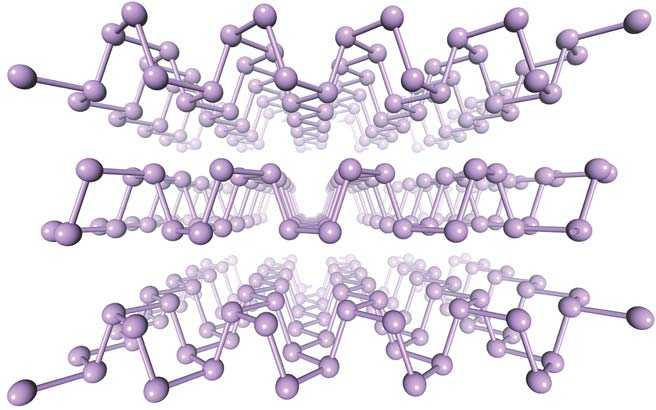
The crystal structure of phosphorus (Credit: Han Liu et al. )
In January of this year, the works of two independent groups, American and Chinese , were published, which managed to make significant progress in obtaining phosphorus. Phosphorous is obtained from the so-called black phosphorus - a layered material similar to graphite, from which graphene is obtained. Black phosphorus has been known since the 1960s, but it was not until 2013 that attempts began to isolate a separate layer from it. In the works in question, black phosphorus was purified to a thickness of two to three atomic layers. Interestingly, as with the first receipt of graphene in 2004, a banal adhesive tape was used to remove excess layers.

The appearance of black phosphorus (Credit: Theodore W. Gray)
Obtaining new materials consisting of a single layer of atoms of various substances has become in recent years one of the most notable areas in materials science. Scientists even dubbed this trend "postgrafen era."
Graphene, which is a single layer of carbon atoms, has unique properties that make it almost ideal for use in electronic devices. In particular, graphene is distinguished by an exceptionally high electron mobility, that is, it conducts electricity well, as well as heat. The problem is that in graphene there is no so-called forbidden zone - the interval of energies that the electron is forbidden to have. The presence of such a zone is extremely desirable, since it is the basis of all modern semiconductor electronics, allowing you to create such important elements as diodes and transistors.
')
That is why the search for substances with a high mobility of electrons, and at the same time with the presence of the forbidden zone, is being actively pursued. Since high electrical conductivity of graphene is largely associated with its two-dimensional, flat structure, new materials are also searched for among those substances that are capable of forming a two-dimensional network. In July 2013, 92 candidates for such materials were found by numerical simulation, but their experimental acquisition turned out to be associated with a large number of difficulties.
Like graphene, phosphorus consists of hexagons, but is not completely flat - some atoms are just above the plane, others - just below. This, however, slightly slows down electrons compared to graphene. At the same time, phosphorus has a forbidden zone, which allows it to conduct current under different conditions, or not.
Another illustration of the crystal structure of phosphorus (Credit: Likai Li et al. )
Despite the fact that to achieve a thickness of one layer, that is, to obtain pure phosphorus, has not yet succeeded, the scientists are full of optimism. For example, it was shown that even in the samples obtained, the velocity of the electrons is comparable with another candidate for “postgrafens” - molybdenum disulfide, consisting of sulfur and molybdenum atoms. Moreover, the presence of only one substance in the structure of phosphorus atoms - phosphorus - and not two, makes the new material more attractive from the point of view of ease of manufacture.
Phosphorous is not the only analogue of graphene, consisting of one kind of atoms. Earlier, it was possible to obtain monatomic layers of silicon, silicon, germanium, and germanium. Both of these materials have a higher electrical conductivity than phosphorus, but just like graphene, they do not have a forbidden zone. Theoretically, a more interesting candidate is the stan - a monatomic tin layer that has both high electron mobility and a band gap, but was predicted only in 2013 and has not yet been obtained by anyone.
A common problem of all the discussed materials is their instability. In the air, they begin to actively oxidize and quickly deteriorate. Special tricks that were able to stabilize the silicon in 2012, still do not allow to use this material in real devices. Phosphorus should be more stable than its competitors, but its production is more difficult: to obtain a black modification, high-purity phosphorus is required to be placed under enormous pressure. The process of further layer removal is also not yet optimized.
In any case, the very possibility of obtaining a two-dimensional material with a forbidden zone is attractive enough to continue research in this area, and potential commercial success promises to cover any time costs.
In preparing the text used materials Nature News .

The crystal structure of phosphorus (Credit: Han Liu et al. )
In January of this year, the works of two independent groups, American and Chinese , were published, which managed to make significant progress in obtaining phosphorus. Phosphorous is obtained from the so-called black phosphorus - a layered material similar to graphite, from which graphene is obtained. Black phosphorus has been known since the 1960s, but it was not until 2013 that attempts began to isolate a separate layer from it. In the works in question, black phosphorus was purified to a thickness of two to three atomic layers. Interestingly, as with the first receipt of graphene in 2004, a banal adhesive tape was used to remove excess layers.

The appearance of black phosphorus (Credit: Theodore W. Gray)
Obtaining new materials consisting of a single layer of atoms of various substances has become in recent years one of the most notable areas in materials science. Scientists even dubbed this trend "postgrafen era."
Graphene, which is a single layer of carbon atoms, has unique properties that make it almost ideal for use in electronic devices. In particular, graphene is distinguished by an exceptionally high electron mobility, that is, it conducts electricity well, as well as heat. The problem is that in graphene there is no so-called forbidden zone - the interval of energies that the electron is forbidden to have. The presence of such a zone is extremely desirable, since it is the basis of all modern semiconductor electronics, allowing you to create such important elements as diodes and transistors.
')
That is why the search for substances with a high mobility of electrons, and at the same time with the presence of the forbidden zone, is being actively pursued. Since high electrical conductivity of graphene is largely associated with its two-dimensional, flat structure, new materials are also searched for among those substances that are capable of forming a two-dimensional network. In July 2013, 92 candidates for such materials were found by numerical simulation, but their experimental acquisition turned out to be associated with a large number of difficulties.
Like graphene, phosphorus consists of hexagons, but is not completely flat - some atoms are just above the plane, others - just below. This, however, slightly slows down electrons compared to graphene. At the same time, phosphorus has a forbidden zone, which allows it to conduct current under different conditions, or not.

Another illustration of the crystal structure of phosphorus (Credit: Likai Li et al. )
Despite the fact that to achieve a thickness of one layer, that is, to obtain pure phosphorus, has not yet succeeded, the scientists are full of optimism. For example, it was shown that even in the samples obtained, the velocity of the electrons is comparable with another candidate for “postgrafens” - molybdenum disulfide, consisting of sulfur and molybdenum atoms. Moreover, the presence of only one substance in the structure of phosphorus atoms - phosphorus - and not two, makes the new material more attractive from the point of view of ease of manufacture.
Phosphorous is not the only analogue of graphene, consisting of one kind of atoms. Earlier, it was possible to obtain monatomic layers of silicon, silicon, germanium, and germanium. Both of these materials have a higher electrical conductivity than phosphorus, but just like graphene, they do not have a forbidden zone. Theoretically, a more interesting candidate is the stan - a monatomic tin layer that has both high electron mobility and a band gap, but was predicted only in 2013 and has not yet been obtained by anyone.
A common problem of all the discussed materials is their instability. In the air, they begin to actively oxidize and quickly deteriorate. Special tricks that were able to stabilize the silicon in 2012, still do not allow to use this material in real devices. Phosphorus should be more stable than its competitors, but its production is more difficult: to obtain a black modification, high-purity phosphorus is required to be placed under enormous pressure. The process of further layer removal is also not yet optimized.
In any case, the very possibility of obtaining a two-dimensional material with a forbidden zone is attractive enough to continue research in this area, and potential commercial success promises to cover any time costs.
In preparing the text used materials Nature News .
Source: https://habr.com/ru/post/212045/
All Articles