Novec ® ® 1230 © ® ™ Pro Applicability for a Submerged Computer
Based on an article about freon for fire fighting: habrahabr.ru/company/3mrussia/blog/200840
A bunch of people appeared in the comments who wanted to use this liquid to create a computer immersed in liquid. I will try to justify why this is a bad idea. The liquid will be called freon, because it is so shorter and in general it is freon. For full perception, it is desirable to remember chemistry and physics in the amount of a little less than the school curriculum, well, or to be able to quickly google. At the same time, I will check my long-standing suspicion that a person educated and able to use the brain can, as a base, master an absolutely extraneous field of knowledge in a very short time. The things described in the post are obvious to me, so somewhere I could miss the key points for understanding. Write in the comments, I will correct. And tell me how to insert Tehov formulas in the post - I will alter the current horror.
The most basic things.
')
1 mole is the amount of a substance that contains 6.02e23 molecules, atoms or some other particles at our discretion. To convert moles to kilograms and back, the molar mass of the substance is used, i.e. the mass of one mole. For freon it is 316.04 g / mol.
The system is the area of space that we are considering. In our case, it can be an aquarium with freon and computer giblets. A phase is an area of a system in which the physical and chemical properties are the same or have a small gradient. The phases are separated by phase boundaries. At the phase boundary, some properties change abruptly. The phase can be incoherent and consist of more than one fragment. Example:

We have such phases: water, ice, glass, air. Ice lies in several pieces, but goes to offset as a single phase, because the composition and physical. the properties of all the pieces are the same.
Shows how much solute is in our solution. The terms solution, solvent, solute, I consider intuitive. Units of measure - for example g / l. Fine point - by default the volume is taken for the whole solution, and not for the solvent. When mixing substances, the volume of the mixture will most likely not be equal to the sum of the volumes of the components. Our solution is always homogeneous, the interfaces between the phases are permissible only with the atmosphere and the walls of the container. For each solvent-solute pair there is almost always a limiting concentration, it is solubility, it is the concentration of a saturated solution. Again, I refer to intuition and do not describe in detail. For example, for food salt NaCl and water, this is 36 g of salt per 100 ml of water at 25 Celsius. If you try to make a solution with a higher concentration, you get a saturated solution, and the excess salt will fall to the bottom. Sometimes solubility is unlimited, and then you can create a solution with any concentration from 0 to 100%. As here, for example:
On the left is also a solution, albeit a solid one. But let's not climb into the jungle. Solubility can be indirectly determined even for almost insoluble substances, it will just be very small.
A little more complicated. Take not one solvent, but two, but those that do not mix with each other. For example, water and oil. Obviously, they will stratify. Now we will add a small amount of our freon there and arrange a shake it baby.
After both liquids stratify back, freon is somehow distributed between solvents, and we get two unsaturated (because freon is very small) solution with different concentrations. The ratio of these concentrations (aqueous solution in the denominator) is called the distribution constant. Usually work with its decimal logarithm. The constant has its own meaning for each pair of solvents, and will change when changing the oil to gasoline. The larger the constant, the greater the tendency of freon to move from water to this solvent.
They are gas mixtures. The most convenient way to express gas content in a mixture in applied calculations is partial pressure. This is the total pressure of the gas mixture multiplied by the volume fraction of gas in the mixture. Example: air pressure is 101.3 kPa, oxygen in it is 21% by volume. Total oxygen partial pressure in air 101.3 * 0.21 = 21.3 kPa.
Exactly one equation of state of an ideal gas named after Mendeleev-Clapeyron:
pV = nRT, where p is the pressure, V is the volume, n is the amount of substance in moles, R is the universal gas constant (8.314 in the SI system), T is the temperature in Kelvin. Substitute the values in the SI system, we consider. The formula applies to partial pressures.
A special case of the distribution of freon in the system liquid-air.
p = k * c, where p is the partial pressure, k is a constant, c is the concentration of a substance in a liquid. For freon, the constant is “large”, as indicated in one of the security documents. This suggests that freon will actively move from water to air.
Take a jar of liquid freon, open it and put it on a table in a hermetically sealed room. He will begin to evaporate. To evaporate the freon, you need to give him some energy - the heat of evaporation. In our case, it can come from the kinetic energy of the molecules of the system. It is spent on the separation of the freon molecule from the total liquid mass and pushing it into the medium of gas. If the freon molecule does not have this energy in the gas phase, then it will most likely stick to the surface of the liquid — condensation will occur. That is, we have two opposite processes - evaporation of a liquid and vapor condensation. In the first approximation, the rate of evaporation increases with an increase in the temperature of the liquid, and the rate of condensation increases with an increase in the partial pressure of freon in the air. Obviously, as freon evaporates, its partial pressure (= its content in the room air) will increase, and the condensation process will accelerate. And then the rate of condensation will be equal to the rate of evaporation, and the system will come into dynamic equilibrium. In the air there will be some stable partial pressure of freon. This is called saturated vapor pressure. It depends only on temperature and grows when heated. When the pressure of saturated vapor is compared with atmospheric - the liquid begins to boil.
Clausius and Clapeyron a century and a half ago derived an equation relating temperature, heat of evaporation, and saturated vapor pressure. In a slightly simplified form, convenient for use, it looks like this:
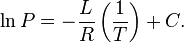
where P is the vapor pressure, L is the heat of evaporation, RT is above, C is the integration constant. We can find it from the condition about the boiling point and saturated vapor pressure equal to the atmospheric one. Total at T = 322 K and L = 88.1 kJ / kg = 27.8 kJ / mol P should be 101.3 kPa, i.e. lnP = 11.53.
Get C = 21.93
Calculate the vapor pressure at room temperature, for example 18C.
P = 33.5 kPa. This is 30% by volume! It is obviously impossible to breathe in this way, well at least nothing will catch fire. This concentration of freon is much greater than necessary to extinguish the flame.
Comments about the sealed enclosure. In this case, the saturated vapor pressure will be summed with the one inside the body besides freon. That is, when sealing the case with air inside at 25 degrees inside will be 1.3 atm, at 49 degrees - 2.0 atm, and so on. This imposes certain requirements on the strength of the walls and sealing. How to account for uneven heating? For example, the processor heats up to 70C, and the motherboard is cold. In this case, there will be local boiling of the liquid and a very rapid temperature equalization due to heat transfer by the freon vapor. So approximately we need to take the average temperature in the hospital, i.e. body volume.
Suppose an extreme case of the love of art. Comp with freon, the room is sealed, the user in the OZK and an insulating gas mask. Take a room 5x4x3 meters, a total volume of 60 cubes, temperature 18C. How much freon will fly into the atmosphere? We substitute the pressure from the previous calculation into the gas state equation, we get n = 830 mol. This is 262 kg or 152 liters. Here is a barrel:

It's just freon, which will quietly hang in the air is not very big room.
Toxicity can be quantified. For this, two sets of parameters are commonly used: MPC and LD50. LD50 - the ability of a substance to cause acute poisoning. This is a single dose, after which 50% of experimental animals die. In this case, the researcher puts an asterisk and indicates in small print with which animals the experiment was and how the substance was injected - with food, water, air, on the skin, through the vein, intraperitoneally, etc. It is usually measured in mg / kg still live weight.
MAC is the maximum permissible concentration of our substance. For her, it stipulates where it is and in what conditions it acts. For example, there may be an MPC in the air of the working area, or there may be an MPC in food. MPC shows the toxicity of a substance with prolonged exposure and indirectly, a tendency to accumulate in the body. Units of measurement in the post-Soviet space are usually mg / kg for food and mg / m3 for air. In English-language literature, people like ppm - part per million, usually by weight. A simple recount shows that 1 ppm = 1 mg / kg. To calm the public, various options of deliberately safe concentrations are used - for example, take the MPC and divide by 100. The consumer is more comfortable with this. It is clear that the LD50 and MPC less, the substance is more toxic. In general, there is no communication between the LD50 and MPC values.
For our MPC freon in the air of the working area is 150 ppm (paragraph 8.3 MSDS ). For comparison with the previous calculation, we will recalculate the vapor pressure: 150 / 1e6 * 101.3 kPa = 15.2 Pa. Or 2000 times less.
No other toxicity data is given. Apparently, the original freon can be considered low toxicity when used as intended, even with small leaks, but there is one nuance. The increased environmental friendliness of this freon is achieved through its instability to ultraviolet. Under UV radiation with a wavelength of about 300 nm, hydrogen fluoride and trifluoroacetic acid are obtained - pieces that are relatively harmless to the ozone layer, but very unpleasant with direct exposure to humans.
Why did I talk about distribution above? Toxicity is often associated with the value of the distribution constant. Cell membranes of living organisms are thin layers of fat. To penetrate through it, the substance must be fat soluble, that is, have a large water-oil distribution constant. The manufacturer does not give these data, but there are ways to estimate them approximately based on the structure of the molecule. And for freon, it must be very large.
Another moment. Environmentally friendly production. I am not an expert here, so I asked for help from organics. It is possible that to get 1 ton of freon, you need to spend 100,500 tons of something extremely toxic. Immediately after receiving the answer I will tell about this side of the question.
There is any offtop in pictures
A bunch of people appeared in the comments who wanted to use this liquid to create a computer immersed in liquid. I will try to justify why this is a bad idea. The liquid will be called freon, because it is so shorter and in general it is freon. For full perception, it is desirable to remember chemistry and physics in the amount of a little less than the school curriculum, well, or to be able to quickly google. At the same time, I will check my long-standing suspicion that a person educated and able to use the brain can, as a base, master an absolutely extraneous field of knowledge in a very short time. The things described in the post are obvious to me, so somewhere I could miss the key points for understanding. Write in the comments, I will correct. And tell me how to insert Tehov formulas in the post - I will alter the current horror.
About volatility, vaporization and other phase transitions
The most basic things.
')
Amount of substance, mole
1 mole is the amount of a substance that contains 6.02e23 molecules, atoms or some other particles at our discretion. To convert moles to kilograms and back, the molar mass of the substance is used, i.e. the mass of one mole. For freon it is 316.04 g / mol.
Thermodynamic system phase
The system is the area of space that we are considering. In our case, it can be an aquarium with freon and computer giblets. A phase is an area of a system in which the physical and chemical properties are the same or have a small gradient. The phases are separated by phase boundaries. At the phase boundary, some properties change abruptly. The phase can be incoherent and consist of more than one fragment. Example:

We have such phases: water, ice, glass, air. Ice lies in several pieces, but goes to offset as a single phase, because the composition and physical. the properties of all the pieces are the same.
Concentration
Shows how much solute is in our solution. The terms solution, solvent, solute, I consider intuitive. Units of measure - for example g / l. Fine point - by default the volume is taken for the whole solution, and not for the solvent. When mixing substances, the volume of the mixture will most likely not be equal to the sum of the volumes of the components. Our solution is always homogeneous, the interfaces between the phases are permissible only with the atmosphere and the walls of the container. For each solvent-solute pair there is almost always a limiting concentration, it is solubility, it is the concentration of a saturated solution. Again, I refer to intuition and do not describe in detail. For example, for food salt NaCl and water, this is 36 g of salt per 100 ml of water at 25 Celsius. If you try to make a solution with a higher concentration, you get a saturated solution, and the excess salt will fall to the bottom. Sometimes solubility is unlimited, and then you can create a solution with any concentration from 0 to 100%. As here, for example:
Hidden text

On the left is also a solution, albeit a solid one. But let's not climb into the jungle. Solubility can be indirectly determined even for almost insoluble substances, it will just be very small.
Distribution
A little more complicated. Take not one solvent, but two, but those that do not mix with each other. For example, water and oil. Obviously, they will stratify. Now we will add a small amount of our freon there and arrange a shake it baby.
Hidden text

After both liquids stratify back, freon is somehow distributed between solvents, and we get two unsaturated (because freon is very small) solution with different concentrations. The ratio of these concentrations (aqueous solution in the denominator) is called the distribution constant. Usually work with its decimal logarithm. The constant has its own meaning for each pair of solvents, and will change when changing the oil to gasoline. The larger the constant, the greater the tendency of freon to move from water to this solvent.
Gas solutions
They are gas mixtures. The most convenient way to express gas content in a mixture in applied calculations is partial pressure. This is the total pressure of the gas mixture multiplied by the volume fraction of gas in the mixture. Example: air pressure is 101.3 kPa, oxygen in it is 21% by volume. Total oxygen partial pressure in air 101.3 * 0.21 = 21.3 kPa.
Calculation of gas parameters
Exactly one equation of state of an ideal gas named after Mendeleev-Clapeyron:
pV = nRT, where p is the pressure, V is the volume, n is the amount of substance in moles, R is the universal gas constant (8.314 in the SI system), T is the temperature in Kelvin. Substitute the values in the SI system, we consider. The formula applies to partial pressures.
Henry's Law
A special case of the distribution of freon in the system liquid-air.
p = k * c, where p is the partial pressure, k is a constant, c is the concentration of a substance in a liquid. For freon, the constant is “large”, as indicated in one of the security documents. This suggests that freon will actively move from water to air.
Evaporation
Take a jar of liquid freon, open it and put it on a table in a hermetically sealed room. He will begin to evaporate. To evaporate the freon, you need to give him some energy - the heat of evaporation. In our case, it can come from the kinetic energy of the molecules of the system. It is spent on the separation of the freon molecule from the total liquid mass and pushing it into the medium of gas. If the freon molecule does not have this energy in the gas phase, then it will most likely stick to the surface of the liquid — condensation will occur. That is, we have two opposite processes - evaporation of a liquid and vapor condensation. In the first approximation, the rate of evaporation increases with an increase in the temperature of the liquid, and the rate of condensation increases with an increase in the partial pressure of freon in the air. Obviously, as freon evaporates, its partial pressure (= its content in the room air) will increase, and the condensation process will accelerate. And then the rate of condensation will be equal to the rate of evaporation, and the system will come into dynamic equilibrium. In the air there will be some stable partial pressure of freon. This is called saturated vapor pressure. It depends only on temperature and grows when heated. When the pressure of saturated vapor is compared with atmospheric - the liquid begins to boil.
Why is all this necessary?
Clausius and Clapeyron a century and a half ago derived an equation relating temperature, heat of evaporation, and saturated vapor pressure. In a slightly simplified form, convenient for use, it looks like this:
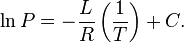
where P is the vapor pressure, L is the heat of evaporation, RT is above, C is the integration constant. We can find it from the condition about the boiling point and saturated vapor pressure equal to the atmospheric one. Total at T = 322 K and L = 88.1 kJ / kg = 27.8 kJ / mol P should be 101.3 kPa, i.e. lnP = 11.53.
Get C = 21.93
Calculate the vapor pressure at room temperature, for example 18C.
P = 33.5 kPa. This is 30% by volume! It is obviously impossible to breathe in this way, well at least nothing will catch fire. This concentration of freon is much greater than necessary to extinguish the flame.
Comments about the sealed enclosure. In this case, the saturated vapor pressure will be summed with the one inside the body besides freon. That is, when sealing the case with air inside at 25 degrees inside will be 1.3 atm, at 49 degrees - 2.0 atm, and so on. This imposes certain requirements on the strength of the walls and sealing. How to account for uneven heating? For example, the processor heats up to 70C, and the motherboard is cold. In this case, there will be local boiling of the liquid and a very rapid temperature equalization due to heat transfer by the freon vapor. So approximately we need to take the average temperature in the hospital, i.e. body volume.
Suppose an extreme case of the love of art. Comp with freon, the room is sealed, the user in the OZK and an insulating gas mask. Take a room 5x4x3 meters, a total volume of 60 cubes, temperature 18C. How much freon will fly into the atmosphere? We substitute the pressure from the previous calculation into the gas state equation, we get n = 830 mol. This is 262 kg or 152 liters. Here is a barrel:

It's just freon, which will quietly hang in the air is not very big room.
Toxicology for beginners
Toxicity can be quantified. For this, two sets of parameters are commonly used: MPC and LD50. LD50 - the ability of a substance to cause acute poisoning. This is a single dose, after which 50% of experimental animals die. In this case, the researcher puts an asterisk and indicates in small print with which animals the experiment was and how the substance was injected - with food, water, air, on the skin, through the vein, intraperitoneally, etc. It is usually measured in mg / kg still live weight.
MAC is the maximum permissible concentration of our substance. For her, it stipulates where it is and in what conditions it acts. For example, there may be an MPC in the air of the working area, or there may be an MPC in food. MPC shows the toxicity of a substance with prolonged exposure and indirectly, a tendency to accumulate in the body. Units of measurement in the post-Soviet space are usually mg / kg for food and mg / m3 for air. In English-language literature, people like ppm - part per million, usually by weight. A simple recount shows that 1 ppm = 1 mg / kg. To calm the public, various options of deliberately safe concentrations are used - for example, take the MPC and divide by 100. The consumer is more comfortable with this. It is clear that the LD50 and MPC less, the substance is more toxic. In general, there is no communication between the LD50 and MPC values.
For our MPC freon in the air of the working area is 150 ppm (paragraph 8.3 MSDS ). For comparison with the previous calculation, we will recalculate the vapor pressure: 150 / 1e6 * 101.3 kPa = 15.2 Pa. Or 2000 times less.
No other toxicity data is given. Apparently, the original freon can be considered low toxicity when used as intended, even with small leaks, but there is one nuance. The increased environmental friendliness of this freon is achieved through its instability to ultraviolet. Under UV radiation with a wavelength of about 300 nm, hydrogen fluoride and trifluoroacetic acid are obtained - pieces that are relatively harmless to the ozone layer, but very unpleasant with direct exposure to humans.
Why did I talk about distribution above? Toxicity is often associated with the value of the distribution constant. Cell membranes of living organisms are thin layers of fat. To penetrate through it, the substance must be fat soluble, that is, have a large water-oil distribution constant. The manufacturer does not give these data, but there are ways to estimate them approximately based on the structure of the molecule. And for freon, it must be very large.
Another moment. Environmentally friendly production. I am not an expert here, so I asked for help from organics. It is possible that to get 1 ton of freon, you need to spend 100,500 tons of something extremely toxic. Immediately after receiving the answer I will tell about this side of the question.
What else can be done with freon
There is any offtop in pictures
Hidden text
Aquarium (or freonrium?) With roborybami


Hidden text
Liquid breathing system


Hidden text
Uber cooler


Source: https://habr.com/ru/post/194268/
All Articles