3D printing of human organs
UPD : Lab owners - Invitro - now on Habré. Included in their corporate blog. Questions may be addressed directly.
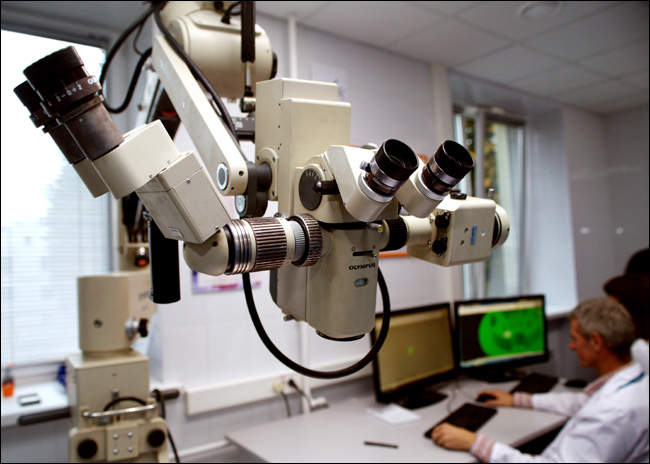
This is from a new laboratory of 3D printing organs. An impressive microscope in front, then you can see two medical engineers behind AutoCAD - they make a layout for the formation of tissue spheroids.
The laboratory of 3D bioprinting of organs (Invitro project) recently opened. A fiery extravaganza of misunderstanding of what is being done is going on around her. In general, although I am not a microbiologist, it became interesting to me. I made my way to the developer - V.A. Mironov. It was he who invented the technology of printing organs and patented it in the USA, participated in the development of already three modifications of bioprinter, and it was he who was “chief in science” in the new laboratory in Moscow:
')

V.A. Mironov (MD, Ph.D., professor with 20 years of experience in microbiology, in particular, on the border with IT) - in the process of an hour and a half explaining to me the essence of the technology, I painted a bunch of paper.
In a few words about the press, he could not tell, because you first need to understand a certain history of the issue. For example, why did we have to discard the bright idea of growing a headless embryo in a surrogate mother, and then remove a kidney from it and place it in the bio-extractor for accelerated maturation.
For now the main thing. Do not rush to drink everything that burns: it is still very far from the new liver . Go.
So, first there was gene therapy : the patient was injected with the appropriate complexes. Certain cells were isolated, the necessary genes were introduced into them, then the cells were placed in the human body. There was not enough insulin - this is the gene that produces its creation. We take the cell complex, modify, inject the patient. The idea is excellent, but with one fundamental flaw: the patient is cured immediately and there is no need to buy anything after the operation. That is, guess who it was across the throat. It was difficult, and then one of the patients died - and the wave of lawsuits and bans, characteristic of the United States, began, resulting in the study having to be curtailed. As a result, there is a method, but not really tested.
The next trend was cell therapy - the use of embryonic stem cells. The method is excellent: “universal” cells are taken, which can be developed to any necessary for the patient. The problem is that to get them somewhere, you need an embryo. The embryo is obviously consumed in the process of obtaining cells. And this is the moral and ethical problem that caused the prohibition of the use of such cells.
Next, tissue engineering is when you take the base, put the cells on it, shove it all into the bioreactor, get the result (organ) that the patient needs. As a prosthesis, only alive. Here is an important point: the main difference from the prosthesis is that the prosthesis is originally from inorganic, and it is unlikely to ever integrate into the body “like a native one”. You can't scratch a wooden leg.
Methods of tissue engineering are skeleton - when used leached (decanted) corpse organ, which is then "populated" by the patient's cells. Other research groups have tried to work with porcine protein frameworks of organs (donor-people are not needed, but immunocompatibility rises to its full height). Frames are artificial - from different materials, some research groups have experimented even with sugar.
Mironov himself practices frameless technology (using a hydrogel as the basis). In his method, the base-polymer quickly degrades and as a result only the cellular material remains. To put it simply, the framework is first inserted from the neo-borders with the cells placed, and then the framework “dissolves”, and the cells of an already grown organ take over its functions. For scaffolds, the same material is used as for surgical sutures: it easily and simply degrades in the human body.
Here the main question is why 3D printing is needed. To understand this, let's dig a little deeper into the existing methods of tissue engineering.
In general, the idea of inserting a pre-grown organic organ into a person is excellent. Let's look at three options for the development of technology:
The obvious complex points of the method are as follows:
So, a 3D printer is just a piece of the line for fabricating organs: it needs to be provided with a drawing, material, and then the resulting model of the organ from the cells is still to be grown. Now let's take a look at the steps how all the problems described above are solved.
So, a CAD file is taken (now - stl format) with an organ model. The easiest way to get a model is to do a three-dimensional scan of the patient himself, and then modify the data with your hands. Now the current constructs are modeled in AutoCAD.

You can see the simulation. The 3D structure is like a regular part - only instead of plastic there will be tissue spheroids.
Material is taken - tissue spheroids, which will be printed. A hydrogel is used as the base, which acts as a connecting structure. Then the 3D printer prints the organ from these tissue spheroids.

The first experiment confirms that a whole organ can be assembled from pieces: scientists have cut a chicken's heart into fragments and have grown it again. Successfully.
Now the question is where to get the cells for this material. The best ones are human embyonic stem cells, of which cells can be made for any tissue by sequential differentiation. But they cannot be touched, as we know. But you can take iPS - induced pluripotent stem cells. They can be made from bone marrow, tooth pulp or the patient’s normal adipose tissue - and produced by various companies around the world.
The scheme is this: a person goes to the clinic, makes liposuction, adipose tissue is frozen and put in the repository. If necessary, it comes out, the necessary cells are made of it (ATDSC, one such complex exists in Russia) and then differentiated by purpose. For example, iPS can be made of fibroblasts, of them the renal epithelium, and then the functional epithelium.
Machines for automatically producing such cells are manufactured by General Electric, for example.

Centrifuge. The first step is the separation of material from adipose tissue.
Balls are formed from these cells in special micro hollows on solid material. A cell suspension is placed in the cavity on the mold, then the cells are spliced, and a ball is formed. More precisely - not very smooth spheroid.
The next problem is that the cells in the cartridges are eager to grow together. Tissue spheroids must be isolated from each other, otherwise they will begin to grow together ahead of time. They need to be encapsulated, and for this is used hyaluronic acid derived from serum. It needs very little - just one of the thinnest layer. She also quickly "leaves" after printing.
Print
The head of a 3D printer has three extruders: two gel nozzles and a device producing tissue spheroids. In the first injector with gel - thrombin, in the second - fibrinogen. Both gels are relatively stable until they touch. But when fibrinogen protein is cleaved by thrombin, fibrin monomer is formed. It is to them as concrete that tissue spheroids are held together. With the depth of the layer corresponding to the diameter of the spheroid, the material can be successively applied row by row — a layer is made, fixed, transferred to the next. Then fibrin easily degrades in the medium and is washed away during perfusion, and only the desired tissue remains.

That's how the tubes will be printed
The printer prints in layers of 250 micrometers: this is the balance between the optimal block size and the risk of hypoxia in the spheroid. For half an hour, you can print a tissue-engineering construction of 10x10 centimeters - but this is not an organ yet, but a tissue-engineering construction, “snot” in the jargon. In order for a structure to become an organ, it must live, have a clear form, carry functions.

A microscope with a huge focal length looks at a glass cube with a 3D printer.

Printhead. While there are tests of the complex on plastic. The printer is now printing consumables, plastic fixtures-molds to create spheroids. At the same time, tests are being performed on a sterile box for a 3D printer with an electronic device running.
The main question is that cells, in general, would not be bad to have access to oxygen and nutrients . Otherwise, they begin, roughly speaking, to rot. When the body is thin, there are no problems, but with a couple of millimeters this is important. True, an elephant, for example, has cartilage up to 5 millimeters - but they are mounted where there is a lot of pressure due to the mass of the rest of the elephant. So, in order for the printed organ not to deteriorate during the fabrication process, microcirculation is needed. This is done by printing these vessels and capillaries, plus using the thinnest perfusion holes made by inorganic instruments (roughly speaking, the construction blocks arrive at the polymer skewer, which is then removed).

Fabric sealing

Tissue combination of several cell types without mixing
The future organ is placed in the bioreactor. This, greatly simplifying, a bank with a controlled environment in which the necessary substances are supplied to the inputs and outputs of the organ, plus accelerated maturation due to the effect of growth factors is ensured.
What is interesting is that the architecture of an organ is usually similar to the usual OOP encapsulated object — the entrance artery, the exit vein — and a bunch of functions inside. It is assumed that the bioreactor will provide the necessary input and output. But this is still a theory, not one has yet been collected. But the project has been worked out to the stage “it is possible to assemble a prototype”.

Hung in the lab. The first stage is seen: obtaining the basic elements, the second - a 3D printer with three extruders, the third - moving away from a prototype to an industrial model, then testing animals, then going to the IPO and setting people.

Entire line - cell sorter, fabric spheroid fabricator, printer, perfusion unit
Now who needs all this on stage, as long as there are no organs themselves.
The first major customers - the military . Actually, as it is not difficult to guess, DARPA goes to visit all scientists involved in this topic. They have two applications - a test (there is a lot that cannot be tested on living people, but I want - a separate organ would be very useful) and a medical one. For example, a democracy fighter tears off his hand, and crawling to the hospital for a day. It would be good to close the hole, take off the pain, give him the opportunity to shoot another 5 hours, and then come to the nurse on his own two feet. In theory, it is possible either robots that collect all this in place, or patches of human tissue, which are already seriously thinking about putting on burns.
The second client is pharma . There, drugs are tested for 15 years before entering the market. As Americans joke, it is easier to kill a colleague than a mouse. On the mouse you need to collect a bunch of documents in hand thick. Certified mice are very expensive as a result. And the results for the animal are different from human ones. Existing models of tests on flat cell models and on animals are not sufficiently basic. In the laboratory, I was told that approximately 7% of new drug formulas in the world did not reach clinical trials because of the nephrotoxicity detected at the stage of preclinical trials. Of those that have reached, about a third have problems with toxicity. That is why, by the way, one of the first tasks is to test the functionality of the nephrons made in the laboratory. Fabrics and organs from the printer will significantly accelerate the development of drugs, and this is a lot of money.
The third client is hospitals. The kidney transplant market with the United States, for example - $ 25 billion. At first, it is supposed to simply sell 3D printers to hospitals so that the patient can get what they need. The next (theoretical) step is the creation of complexes for printing organs right inside the patient. The fact is that a miniature print head inside a patient is often much easier to deliver than a large organ. But it is still a dream, although the necessary robots exist.

This is how it should work.
Yes, there is another important topic: in parallel, studies are being conducted on the management of tissue spheroids due to magnetic levitation. The first experiments were simple - iron “nano-saws” were thrust into the fabric, and the spheroids actually flew as they should in the magnet field and were delivered at the site. But differentiation suffered. With sawdust difficult to perform the necessary functions. The next logical step is the metal in the encapsulating layer. But even cooler - micro-capsules with magnetic particles. These scapolds cover a spheroid and can still act as a frame-connector, rising immediately in place, which gives a huge scope for the operational printing of organs.
- Company on Skolkovo
- About the Russian conference on regenerative medicine that this team was doing

A pile of paper, which Mironov izrisoval during the story. Handwriting like a doctor :)

This is from a new laboratory of 3D printing organs. An impressive microscope in front, then you can see two medical engineers behind AutoCAD - they make a layout for the formation of tissue spheroids.
The laboratory of 3D bioprinting of organs (Invitro project) recently opened. A fiery extravaganza of misunderstanding of what is being done is going on around her. In general, although I am not a microbiologist, it became interesting to me. I made my way to the developer - V.A. Mironov. It was he who invented the technology of printing organs and patented it in the USA, participated in the development of already three modifications of bioprinter, and it was he who was “chief in science” in the new laboratory in Moscow:
')

V.A. Mironov (MD, Ph.D., professor with 20 years of experience in microbiology, in particular, on the border with IT) - in the process of an hour and a half explaining to me the essence of the technology, I painted a bunch of paper.
In a few words about the press, he could not tell, because you first need to understand a certain history of the issue. For example, why did we have to discard the bright idea of growing a headless embryo in a surrogate mother, and then remove a kidney from it and place it in the bio-extractor for accelerated maturation.
For now the main thing. Do not rush to drink everything that burns: it is still very far from the new liver . Go.
Evolution of methods
So, first there was gene therapy : the patient was injected with the appropriate complexes. Certain cells were isolated, the necessary genes were introduced into them, then the cells were placed in the human body. There was not enough insulin - this is the gene that produces its creation. We take the cell complex, modify, inject the patient. The idea is excellent, but with one fundamental flaw: the patient is cured immediately and there is no need to buy anything after the operation. That is, guess who it was across the throat. It was difficult, and then one of the patients died - and the wave of lawsuits and bans, characteristic of the United States, began, resulting in the study having to be curtailed. As a result, there is a method, but not really tested.
The next trend was cell therapy - the use of embryonic stem cells. The method is excellent: “universal” cells are taken, which can be developed to any necessary for the patient. The problem is that to get them somewhere, you need an embryo. The embryo is obviously consumed in the process of obtaining cells. And this is the moral and ethical problem that caused the prohibition of the use of such cells.
Next, tissue engineering is when you take the base, put the cells on it, shove it all into the bioreactor, get the result (organ) that the patient needs. As a prosthesis, only alive. Here is an important point: the main difference from the prosthesis is that the prosthesis is originally from inorganic, and it is unlikely to ever integrate into the body “like a native one”. You can't scratch a wooden leg.
Methods of tissue engineering are skeleton - when used leached (decanted) corpse organ, which is then "populated" by the patient's cells. Other research groups have tried to work with porcine protein frameworks of organs (donor-people are not needed, but immunocompatibility rises to its full height). Frames are artificial - from different materials, some research groups have experimented even with sugar.
Mironov himself practices frameless technology (using a hydrogel as the basis). In his method, the base-polymer quickly degrades and as a result only the cellular material remains. To put it simply, the framework is first inserted from the neo-borders with the cells placed, and then the framework “dissolves”, and the cells of an already grown organ take over its functions. For scaffolds, the same material is used as for surgical sutures: it easily and simply degrades in the human body.
Here the main question is why 3D printing is needed. To understand this, let's dig a little deeper into the existing methods of tissue engineering.
Approaching the goal
In general, the idea of inserting a pre-grown organic organ into a person is excellent. Let's look at three options for the development of technology:
- You take the skeleton from the inorganics, sow it with cells - and get a ready-made organ . The method is rough, but working. It is about him that is spoken in most cases when they say “we have printed the organ”. The problem is that somewhere you need to take the "building material" - the cells themselves. And if they are, then it is foolish to use some kind of external framework, when there is an opportunity to simply assemble an organ from them. But the most painful problem is incomplete endothelialization. For example, for the bronchi made so, the level is about 70%. This means that the surface vessels are thrombogenic — by curing a patient, you immediately introduce a new disease to him. Then he must live on heparin or other drugs, or wait for a thrombus and embolism to form. And here the lawyers of the USA, who are ready to play according to the old scenario, are already looking forward to. And the problem of endothelization has not yet been resolved. A possible option is to isolate the progenitor cells of the bone marrow with the help of mobilization with special preparations and homing on the organ, but this is still very far from the practice of fantasy.
- The second method is extremely original and very pleased with its cynicism . Take the cell (fibroblast) of the patient, add 4 genes. Put the resulting cell in a blastocyst (animal embryo) and begin to grow animals. It turns out, for example, a pig with a human pancreas - the so-called chimera. The organ is completely “native”, only the entire infrastructure around it is blood vessels, tissues, and so on, from the pig. And they will be rejected. But nothing. We take a pig, we cut out the body we need (the pig is completely consumed), and then we remove all the pork tissue with a special treatment - it turns out that the organic framework of the body can be used to grow a new one. Some researchers went further and proposed the following lafhak: let's replace the pig with a surrogate mother. Here's how: in addition to 4 genes, one more cell is added that is responsible for acephaly (absence of the head). A surrogate mother is hired who is carrying our mutual embryo friend. It develops without a head, it is good at acephals. Then - ultrasound, finding out that the child turns out to be inferior, and legally permitted abortion. No head - no man, so we did not kill anyone. And here - time! - here we have a theoretically legal biomaterial with undeveloped patient organs. Quickly implant them! Of the obvious drawbacks - well, apart from the moral side - organizational complexity and possible legal complications in the future.
- And finally, there is a third method, about which there is a speech . He is the most modern - three-dimensional printing of organs. And they are engaged in it in a new laboratory. The meaning is this: inorganic scaffolds are not needed (the cells themselves perfectly keep themselves), no need to take organs from someone. The patient gives a little of his adipose tissue (everyone has it, only skinny Japanese complained during the experiments), the necessary structural elements are obtained from it by sequential processing of the cells. A three-dimensional model of the organ is created, converted into a CAD file, then this one is given to a 3D printer, which can print with our cells and understands at what point in the three-dimensional space it needs to “lay down” a specific cell type. At the exit - a tissue construct, which must be placed in a special environment, until the problems with hypoxia began. In the biorecator, the tissue construct "matures". Then the organ can be “transplanted” to the patient.
The obvious complex points of the method are as follows:
- Getting a model of the body. We need somewhere to take the scheme. It is quite simple.
- Getting the cells themselves. Obviously, we need material to print the body.
- Build a printer so that the cells can be printed (a bunch of problems with the formation of the structure of the body).
- Hypoxia (lack of oxygen) during the creation of an organ.
- Realization of nutrition of the body and its maturation until readiness.
So, a 3D printer is just a piece of the line for fabricating organs: it needs to be provided with a drawing, material, and then the resulting model of the organ from the cells is still to be grown. Now let's take a look at the steps how all the problems described above are solved.
Organ model
So, a CAD file is taken (now - stl format) with an organ model. The easiest way to get a model is to do a three-dimensional scan of the patient himself, and then modify the data with your hands. Now the current constructs are modeled in AutoCAD.

You can see the simulation. The 3D structure is like a regular part - only instead of plastic there will be tissue spheroids.
Material
Material is taken - tissue spheroids, which will be printed. A hydrogel is used as the base, which acts as a connecting structure. Then the 3D printer prints the organ from these tissue spheroids.

The first experiment confirms that a whole organ can be assembled from pieces: scientists have cut a chicken's heart into fragments and have grown it again. Successfully.
Now the question is where to get the cells for this material. The best ones are human embyonic stem cells, of which cells can be made for any tissue by sequential differentiation. But they cannot be touched, as we know. But you can take iPS - induced pluripotent stem cells. They can be made from bone marrow, tooth pulp or the patient’s normal adipose tissue - and produced by various companies around the world.
The scheme is this: a person goes to the clinic, makes liposuction, adipose tissue is frozen and put in the repository. If necessary, it comes out, the necessary cells are made of it (ATDSC, one such complex exists in Russia) and then differentiated by purpose. For example, iPS can be made of fibroblasts, of them the renal epithelium, and then the functional epithelium.
Machines for automatically producing such cells are manufactured by General Electric, for example.

Centrifuge. The first step is the separation of material from adipose tissue.
Balls are formed from these cells in special micro hollows on solid material. A cell suspension is placed in the cavity on the mold, then the cells are spliced, and a ball is formed. More precisely - not very smooth spheroid.
Processing of building blocks
The next problem is that the cells in the cartridges are eager to grow together. Tissue spheroids must be isolated from each other, otherwise they will begin to grow together ahead of time. They need to be encapsulated, and for this is used hyaluronic acid derived from serum. It needs very little - just one of the thinnest layer. She also quickly "leaves" after printing.
The head of a 3D printer has three extruders: two gel nozzles and a device producing tissue spheroids. In the first injector with gel - thrombin, in the second - fibrinogen. Both gels are relatively stable until they touch. But when fibrinogen protein is cleaved by thrombin, fibrin monomer is formed. It is to them as concrete that tissue spheroids are held together. With the depth of the layer corresponding to the diameter of the spheroid, the material can be successively applied row by row — a layer is made, fixed, transferred to the next. Then fibrin easily degrades in the medium and is washed away during perfusion, and only the desired tissue remains.

That's how the tubes will be printed
The printer prints in layers of 250 micrometers: this is the balance between the optimal block size and the risk of hypoxia in the spheroid. For half an hour, you can print a tissue-engineering construction of 10x10 centimeters - but this is not an organ yet, but a tissue-engineering construction, “snot” in the jargon. In order for a structure to become an organ, it must live, have a clear form, carry functions.

A microscope with a huge focal length looks at a glass cube with a 3D printer.

Printhead. While there are tests of the complex on plastic. The printer is now printing consumables, plastic fixtures-molds to create spheroids. At the same time, tests are being performed on a sterile box for a 3D printer with an electronic device running.
Post processing
The main question is that cells, in general, would not be bad to have access to oxygen and nutrients . Otherwise, they begin, roughly speaking, to rot. When the body is thin, there are no problems, but with a couple of millimeters this is important. True, an elephant, for example, has cartilage up to 5 millimeters - but they are mounted where there is a lot of pressure due to the mass of the rest of the elephant. So, in order for the printed organ not to deteriorate during the fabrication process, microcirculation is needed. This is done by printing these vessels and capillaries, plus using the thinnest perfusion holes made by inorganic instruments (roughly speaking, the construction blocks arrive at the polymer skewer, which is then removed).

Fabric sealing

Tissue combination of several cell types without mixing
The future organ is placed in the bioreactor. This, greatly simplifying, a bank with a controlled environment in which the necessary substances are supplied to the inputs and outputs of the organ, plus accelerated maturation due to the effect of growth factors is ensured.
What is interesting is that the architecture of an organ is usually similar to the usual OOP encapsulated object — the entrance artery, the exit vein — and a bunch of functions inside. It is assumed that the bioreactor will provide the necessary input and output. But this is still a theory, not one has yet been collected. But the project has been worked out to the stage “it is possible to assemble a prototype”.

Hung in the lab. The first stage is seen: obtaining the basic elements, the second - a 3D printer with three extruders, the third - moving away from a prototype to an industrial model, then testing animals, then going to the IPO and setting people.

Entire line - cell sorter, fabric spheroid fabricator, printer, perfusion unit
Markets
Now who needs all this on stage, as long as there are no organs themselves.
The first major customers - the military . Actually, as it is not difficult to guess, DARPA goes to visit all scientists involved in this topic. They have two applications - a test (there is a lot that cannot be tested on living people, but I want - a separate organ would be very useful) and a medical one. For example, a democracy fighter tears off his hand, and crawling to the hospital for a day. It would be good to close the hole, take off the pain, give him the opportunity to shoot another 5 hours, and then come to the nurse on his own two feet. In theory, it is possible either robots that collect all this in place, or patches of human tissue, which are already seriously thinking about putting on burns.
The second client is pharma . There, drugs are tested for 15 years before entering the market. As Americans joke, it is easier to kill a colleague than a mouse. On the mouse you need to collect a bunch of documents in hand thick. Certified mice are very expensive as a result. And the results for the animal are different from human ones. Existing models of tests on flat cell models and on animals are not sufficiently basic. In the laboratory, I was told that approximately 7% of new drug formulas in the world did not reach clinical trials because of the nephrotoxicity detected at the stage of preclinical trials. Of those that have reached, about a third have problems with toxicity. That is why, by the way, one of the first tasks is to test the functionality of the nephrons made in the laboratory. Fabrics and organs from the printer will significantly accelerate the development of drugs, and this is a lot of money.
The third client is hospitals. The kidney transplant market with the United States, for example - $ 25 billion. At first, it is supposed to simply sell 3D printers to hospitals so that the patient can get what they need. The next (theoretical) step is the creation of complexes for printing organs right inside the patient. The fact is that a miniature print head inside a patient is often much easier to deliver than a large organ. But it is still a dream, although the necessary robots exist.

This is how it should work.
Yes, there is another important topic: in parallel, studies are being conducted on the management of tissue spheroids due to magnetic levitation. The first experiments were simple - iron “nano-saws” were thrust into the fabric, and the spheroids actually flew as they should in the magnet field and were delivered at the site. But differentiation suffered. With sawdust difficult to perform the necessary functions. The next logical step is the metal in the encapsulating layer. But even cooler - micro-capsules with magnetic particles. These scapolds cover a spheroid and can still act as a frame-connector, rising immediately in place, which gives a huge scope for the operational printing of organs.
Links
- Company on Skolkovo
- About the Russian conference on regenerative medicine that this team was doing
Bundle of links in English, which tells about the gradual progress:
www.ncbi.nlm.nih.gov/pubmed/12679063
www.ncbi.nlm.nih.gov/pubmed/11500808
www.ncbi.nlm.nih.gov/pubmed/20603872
www.ncbi.nlm.nih.gov/pubmed/21419621
www.ncbi.nlm.nih.gov/pubmed/18423666
www.ncbi.nlm.nih.gov/pubmed/16805712
www.ncbi.nlm.nih.gov/pubmed/15174961
www.ncbi.nlm.nih.gov/pubmed/18333793
www.ncbi.nlm.nih.gov/pubmed/18194071
www.ncbi.nlm.nih.gov/pubmed/18154465
www.ncbi.nlm.nih.gov/pubmed/14981244
www.ncbi.nlm.nih.gov/pubmed/20811099
www.ncbi.nlm.nih.gov/pubmed/19176247
www.ncbi.nlm.nih.gov/pubmed/12740943
www.ncbi.nlm.nih.gov/pubmed/11500808
www.ncbi.nlm.nih.gov/pubmed/20603872
www.ncbi.nlm.nih.gov/pubmed/21419621
www.ncbi.nlm.nih.gov/pubmed/18423666
www.ncbi.nlm.nih.gov/pubmed/16805712
www.ncbi.nlm.nih.gov/pubmed/15174961
www.ncbi.nlm.nih.gov/pubmed/18333793
www.ncbi.nlm.nih.gov/pubmed/18194071
www.ncbi.nlm.nih.gov/pubmed/18154465
www.ncbi.nlm.nih.gov/pubmed/14981244
www.ncbi.nlm.nih.gov/pubmed/20811099
www.ncbi.nlm.nih.gov/pubmed/19176247
www.ncbi.nlm.nih.gov/pubmed/12740943

A pile of paper, which Mironov izrisoval during the story. Handwriting like a doctor :)
Important facts
- Not a single organ printed on a 3D printer has yet been implanted in humans. But there are about a dozen different cases of successful "installation" of such organs in animals.
- Mironov has already collected three existing 3D bioprinter: 2 in Canada, some in Brazil. New in Russia should be the best of all.
- When spheroids are spliced, the tissue is compacted — for example, the kidney will have to be printed three times more than it will be inside the patient — already at the last stage of fabrication, it will become normal size.
- Now they have learned to do basic things, for example, tubes of different types of fabric. After checking the functionality of the cells, it is possible to make complex constructions. For example, a nephron is easily obtained from tubules, and a kidney from a variety of nephrons.
- Robots are needed. In the bronchi, for example, 10 orders of branching — it is somewhat tiring to collect it with your hands, and the patient is not ready to wait thousands of years. The future of fast printing technology is microfluidic extruders that make up to 10 thousand drops per second. Together with a fast robot, they can give an excellent effect.
- The printed organs are immediately atrombogenic — for example, the vessels are immediately lined from the inside with endothelium. This is a very cool advantage: the patient does not risk, and he will not have to sit on pills all his life.
- Checkpoints for a nearby perspective: patents in the Russian Federation, a fully assembled printer, an article in Science or Nature. An international team of scientists has already been assembled, which includes: Doctor of Biological Sciences, Candidate of Biological Sciences, Candidate of Medical Sciences, Doctor of Ph.D.
- The first kidney will be in the 2030th year. It will first cost as a space, but with the scaling of technology - at times cheaper than foreign organs for transplant now.
Source: https://habr.com/ru/post/194064/
All Articles