DIY oxy-acetylene welding
 The path to IT is very thorny for everyone. For example, in my childhood I wanted to be a welder - it's so beautiful when splashes of molten metal fly around! But somehow it didn’t work out: I started to write out the magazine “Young Technician”, where on the last page of one of the numbers they were talking about a robot controlled by the BK-0010 computer ... But the fad remained ...
The path to IT is very thorny for everyone. For example, in my childhood I wanted to be a welder - it's so beautiful when splashes of molten metal fly around! But somehow it didn’t work out: I started to write out the magazine “Young Technician”, where on the last page of one of the numbers they were talking about a robot controlled by the BK-0010 computer ... But the fad remained ...Also, someone probably remembers the transfer of "crazy hands", where various creative (as they would say now) things were made of plastic bottles.
Under the cut, I'll show you how to make a real oxygen-acetylene welding from a plastic bottle, an insulin syringe, a few meters of a rubber hose, a glue gun (where you can go without it) and some other things that can be found in every home *.
')
* In every BarsMonster home.
Theory
 The flame temperature depends on the heat of combustion of the fuel and the heat capacity of the reaction products. When we burn something in the air, we also have to heat the nitrogen (which is almost 80%), so the flame temperature in the air is usually not high (~ 1500-2000 ° C and lower). But in pure oxygen, with the correct ratio of the volume of fuel and oxygen, only the reaction products need to be heated, and much higher temperatures are achievable.
The flame temperature depends on the heat of combustion of the fuel and the heat capacity of the reaction products. When we burn something in the air, we also have to heat the nitrogen (which is almost 80%), so the flame temperature in the air is usually not high (~ 1500-2000 ° C and lower). But in pure oxygen, with the correct ratio of the volume of fuel and oxygen, only the reaction products need to be heated, and much higher temperatures are achievable.Hydrocarbons are usually considered a fuel. Carbon when burned gives carbon dioxide, and hydrogen - water. Water has a very large heat capacity (4.183 vs. 1.4 kJ / (kg * K)), respectively, the more carbon there is in the fuel, and less hydrogen — the higher the potentially attainable temperature in the first approximation.
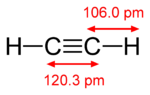
The best combination is for acetylene C 2 H 2 , for example, for methane CH 4 and propane C 3 H 8 - this ratio is much worse.
 But there are other compounds with an equal amount of carbon and hydrogen - for example, benzene, C 6 H 6 . In addition to the toxicity of benzene, less energy is released during its combustion, because in acetylene, “excess” energy is stored in an unstable triple carbon bond, which provides it with one of the highest burning temperatures in oxygen — 3150 ° C.
But there are other compounds with an equal amount of carbon and hydrogen - for example, benzene, C 6 H 6 . In addition to the toxicity of benzene, less energy is released during its combustion, because in acetylene, “excess” energy is stored in an unstable triple carbon bond, which provides it with one of the highest burning temperatures in oxygen — 3150 ° C.This excess energy (~ 16%) can be released during the spontaneous detonation of compressed acetylene even without air (just benzene and vinyl acetylene will be the reaction product). Wikipedia claims that this requires a pressure of only 2 atmospheres - but I squeezed acetylene to 4-5 atmospheres in a syringe and nothing happened (apparently catalysts, shock or elevated temperature are needed). In any case, because of this effect, acetylene is not stored in compressed form, but is dissolved in cylinders in acetone. But there is a simpler and safer method for producing acetylene with small volumes - the reaction of calcium carbide with water. This method will be used.
Remarkably, even higher temperatures can be achieved - if hydrogen-free substances are used as fuels: cyanogen (hi Android), (CN) 2 - burns at 4525 ° C and dicyanoacetylene C 4 N 2 , burns at 4990 ° C (again due to triple carbon bonds, and a smaller relative amount of excess nitrogen). But practically for this purpose they are not used due to toxicity.
Security
Compressed oxygen and acetylene in cylinders - can be very dangerous at the slightest violation of the rules of operation, because of course I will not use them.Acetylene will be generated from a small amount of calcium carbide (~ 100g per session), in a 0.5 l bottle. Initially, I wanted to use 2n to make the pressure more uniform — but after watching YouTube on how a liter of acetylene with oxygen explodes , I decided to cut the sturgeon. In order not to create a dangerous pressure in the generator - the output of acetylene on the burner should never be blocked. The acetylene generator needs to be cooled - otherwise it will be “self-dispersing” of the reaction due to heating.
Oxygen will be generated by a medical oxygen concentrator, which is relatively safe.
There could still be a danger of pumping oxygen into the acetylene generator with subsequent cotton - but for this it is necessary that the protective valve in the oxygen generator does not work and the gas outlet from the burner is blocked (for example, with dirt).
And of course you need to work in special glasses - not only to protect against metal splashes, but also ultraviolet radiation of the flame (that is, transparent plastic protective glasses will not do here).
In order to prevent the accumulation of explosive concentrations of acetylene in the event of leaks - the fan constantly blew the workplace + all operations were carried out in the open air.
There is also the problem of “reverse shock”: when the gas flow rate in the burner becomes too small, the flame goes inside the burner with a pop, and if there is air in acetylene, the flame can reach the acetylene generator. Therefore, I did not set fire to acetylene immediately after the start of the reaction, but waited ~ 15-30 seconds until the air was expelled. Also, this problem can be solved by adding a water valve in the acetylene path.
Design
So, we need an oxygen generator. In my case, the Atmung medical oxygen concentrator (the price is about 20k rubles - but, fortunately, it was already available). It can generate 1 liter per minute of 95% oxygen, and larger volumes at lower concentrations. It works on the principle of short-cycle adsorption without heat due to the different rate of passage of gases through the pores of the zeolite:
Next - the standard acetylene torch "Baby", she has the smallest nozzle, bought in the online store (960 rubles):

My acetylene generator works as follows: water from a can, standing at a height of 1-2 meters (to create pressure) through a needle of an insulin syringe, drops into small drops on calcium carbide in a bottle. As soon as the pressure rises due to the released gas, the water stops dropping until the pressure drops. Thus the system stabilizes itself. However, the generator in a cold water can - to prevent excessive heating:

Result
The flame of acetylene in the air strongly smokes, and looks quite ordinary:
With the inclusion of oxygen, everything changes:

You can melt and set fire to steel, cut all the same not enough power (you need to take a thicker tip, increase the pressure):

It turned out that a flexible glass “optical fiber” is obtained automagically — when molten glass drips, as soon as the thickness of the neck becomes sufficiently small, it cools down very quickly and does not further thin out.

You can melt the glass as oil, sealed capsules of glass tubes:

The task of life is done, I hope you were interested :-)
Ps. And do not repeat it at home.
Addition from the expert (@freuser):
From the point of view of a professional welder (30 years, 11 years of service, of which 2 are gas welding):
The article is generally correct. It should be added that work is being carried out on fireproof surfaces (sparks fly about 2 meters from the wind, and drops of metal, even darkened to ordinary colors, can burn shoes if it is a shoe.)
The design of the generator is called VK (water to carbide), there is also KV and BB (it is googled with diagrams, the copyright is still Soviet :)).
There are no comments to the video, and there’s nothing to watch (from my point of view), but it’s worth adding that large glasses (or whole bottles), as well as stone / concrete / some bricks, when heated, can burst / stratify to form low-flying fragments, which are wonderful they stick in and melt into the skin (especially on the face), however, by a millimeter, not more, and can be easily removed from there.
I would also like to answer precisely habrahabr.ru/post/185720/#comment_6461342 : this is not a back kick, or rather not something that Nepherhotep warned against, but simply a burner or overheated, or rather from low pressure and an obstacle close to the nozzle ( or a blockage inside the nozzle) the flame went towards the flow to the injector (in this burner it is under the cap nut, between it and the valves), but it did not move further. And usually, a kickback is a case when the flame penetrated the injector and went along the hose towards the source. There are two types of backward strikes (I personally observed one): the flame goes through the acetylene hose (normal burning, only the end of the hose constantly burns and the flame moves evenly to the cylinder / generator) and the oxygen one (everything is more beautiful - the hose is suddenly 20-30 cm a piece flashes and turns into tatters, the second pause is the next segment, etc. to the cylinder itself.) Although the second case is rare. The simplest protection is to clamp the hose in the distance, press down with your foot (do not forget about the shoes) and shout your partner “Sanka, close the cylinders, *** !!” For more civil protection, you can make water closures - also a bottle, two tubes, one to the bottom - incoming, the second short - to the burner. Up to half of it is poured with water and all, the bubbles run beautifully))
Source: https://habr.com/ru/post/185720/
All Articles