Currently, the memory effect is also found in lithium-ion batteries.
This is a translation of the article Memory effect now also found in lithium-ion batteries , posted by scientists on the official website of the Institute. Recently, there was news that a memory effect was detected in lithium-ion batteries. After reviewing the information in more detail, I did not find anything sensible, except for short news (in Russian). Therefore, I cite the translation of the article from the official site.
Lithium-ion batteries are high-performance energy storage devices used in many electrical appliances. They can store large amounts of energy in a relatively small volume. Previously it was widely believed that they did not have a memory effect. So experts call the deviation in the operating voltage of the battery, caused by incomplete charging or discharging, as a result of which only part of the stored energy is available, as well as the impossibility of accurately determining the level of battery charge. Scientists from the Paul Scherrer Institute , together with colleagues from the Toyota research laboratory in Japan , have now found that a widely used type of lithium-ion battery has a memory effect. This discovery is particularly important in the use of lithium-ion batteries in the electric vehicle market. The work was published on April 14, 2013 in the scientific journal Nature Materials.
Many of our everyday battery-powered devices are not always smart, as advertised, often have a memory effect. For example, electric shavers or electric toothbrushes, which are charged before they are completely discharged, can be avenged on the user in the future. Batteries remember that you used only part of their capacity - and, in the end, they no longer give out their full energy. Experts call this the "memory effect", which is explained by the fact that the battery's operating voltage drops over time due to incomplete charge-discharge cycles. This means that, although the battery is not yet discharged, the voltage it delivers is sometimes too low to keep the device in working condition. Therefore, the memory effect has two negative consequences: firstly, the useful capacity of the battery decreases, and secondly the correlation between voltage and charge state shifts, so that the latter cannot be reliably determined on the basis of voltage. It has long been known that the memory effect exists in nickel-cadmium and nickel-metal hydride batteries. Since lithium-ion batteries began to be sold successfully in the 1990s, the existence of a memory effect in this type of battery has been ruled out. This new study shows that the view was wrong.

The memory effect and abnormalities associated with abnormal operating voltage have already been confirmed on one of the most common materials used as a positive electrode in lithium-ion batteries, lithium-iron phosphate (LiFePO4). With lithium iron phosphate, the voltage remains almost unchanged over a wide range of state of charge. This means that even a small anomaly in the operating voltage can be misinterpreted (as a significant change in charge). Or, to put it differently: when the state of charge is determined by the voltage, a large error may be caused by a slight variation in voltage. The existence of the memory effect is especially relevant when considering the use of lithium-ion batteries in the electric transport sector. In hybrid cars, in particular, the effect may occur due to the many charge / discharge cycles that occur during normal operation. In such vehicles, the battery is partially recharged during engine braking and when operating in generator mode. And in turn, it is discharged, and usually only partially, during the acceleration phase. Numerous successive cycles of partial charging and discharging lead to the addition of separate small memory effects to a larger memory effect, as this new study demonstrates. This leads to an error in estimating the current state of charge of the battery, in the case where the state of charge is calculated based on the current voltage value.
Researchers identify the microscopic mechanism that occurs during the charge and discharge process as the main cause of the memory effect in lithium-ion batteries. The electrode material - in this case, iron lithium phosphate (LiFePO4) - consists of a large number of small, micron-sized particles, which are charged and discharged separately one after another. Researchers refer to this charging and discharging model as the "many particles model". Particle charges after particle and includes the release of lithium ions. A fully charged particle does not contain lithium ions (lithium-free) and consists only of iron phosphate (FePO4). The discharge, in turn, involves the re-incorporation of lithium atoms into the particles, so that iron phosphate (FePO4) again becomes iron lithium phosphate (LiFePO 4). Changing the amount of lithium associated with charging and discharging causes a change in the chemical potential of individual particles, which leads to a change in the voltage of the battery. However, charging and discharging are not linear processes. During charging, the chemical potential first increases, with the progressive release of lithium ions. But then, the particles reach the critical lithium content (lithium-content), and, accordingly, the chemical potential. At this point, a sharp transition occurs: the particles discard their remaining lithium ions very quickly, but do not change their chemical potential. This transitional period explains why the battery voltage remains almost unchanged in a wide area (voltage plateau).
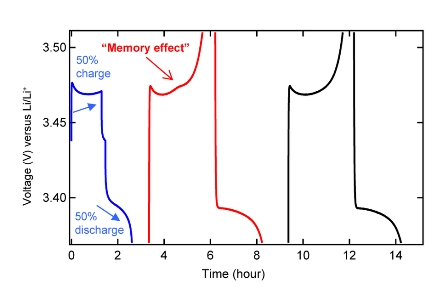
The existence of this potential barrier is vital for the manifestation of the memory effect. As soon as the first particles overcame the potential barrier, and became lithium-free, the electrode particles split into two groups. In other words, at present there is a clear distinction between lithium-rich and lithium-poor particles. If the battery is not fully charged, a certain amount of lithium-rich particles that have not crossed the barrier will return. These particles do not remain on the edge of the barrier for a long time, because this state is unstable, and they will “slide down the slope,” that is, their chemical potential will decrease. Even when the battery is discharged again and all the particles will go to the "rest" before the barrier, this division into two groups will be preserved. An important point: during the next charging process, the first group (lithium-poor particles) will overcome the barrier of the first, while the second group (lithium-rich) will “lag behind”. In order for the “deferred” group to overcome the barrier, their chemical potential must be increased, and this is what causes the overvoltage (“bump” on the graph) characterizing the memory effect. Thus, the memory effect is a consequence of the separation of a population of particles into two groups, with very different concentrations of lithium, which follow the "jumping" of particles across a potential barrier, one after the other. This overvoltage, through which the effect is noticeable, is equal to the additional work that must be performed to transport lagging particles through the potential barrier, after a partial charge.
The time that passes between charging and discharging the battery plays an important role in determining the state of the battery at the end of these processes. Charging and discharging are processes that change the thermodynamic equilibrium of a battery, and this equilibrium can be achieved after a while. Scientists have discovered that a sufficiently long idle can be used to remove the memory effect. However, according to the multi-particle model, this happens only under certain conditions. The memory effect will disappear only with a sufficiently long break between the partial charge cycles and the subsequent full discharge. In such cases, the groups of particles are still separated after a full discharge, but are on the same side of the potential barrier. Thus, the separation will disappear, since the particles reach an equilibrium state in which they all will have the same lithium content. To prevent the memory effect, you must wait after partial charging and before partial discharge. In this case, the particles will be on opposite sides of the potential barrier, this will prevent their reverse separation into “lithium-rich” and “lithium-poor”.
')
According to Petr Novak, the head of the Electrochemical Energy Storage Section at PSI (Electrochemical Energy Storage Section) and co-author of the publication, the study refutes the well-established misconception: battery powered. These were just suggestions that there would be no similar effect. ” To gain knowledge through research, a combination of reflection and hard work is often fruitful: “Our search results consist of a combination of critical research and careful observation. The effect is actually tiny: the relative voltage deviation is only a few particles per thousand. But the key was the idea of finding it at all. Normal battery tests typically examine complete, rather than partial, charge / discharge cycles.
However, this recent discovery is not the last word for the future use of lithium-ion batteries in automobiles. It is really quite possible that the effect can be detected and will be taken into account through smart software adaptation in battery management systems. If this proves successful, the memory effect will not stand in the way of safe and reliable use of lithium-ion batteries in electric vehicles. So now, engineers are faced with the problem of finding the right treatment with a kind of battery memory.
Text: Leonid Leiva
Following the many particle model described here, it is assumed that charging and discharging the battery occurs particle by particle. In this context, by particles, we mean a kind of "grain". This means that the material (LiFePO4) is not one, but rather consists of a set of granules, the crystal structure of which is the same, but the granules have small differences in size, shape or orientation. This is a typical powder structure. From a technical point of view, they are called "crystallites". It can be imagined as approximately the same cubes in size lying next to each other. Each cube will be slightly rotated relative to its neighbors, that is, the cubes are not strictly aligned, but the crystal structure (hexagon shape) is the same for all.
PS
Thank you Mithgol for an invite.
From the translator: Some sentences are very difficult to understand (when read from the first time), I tried to reformulate and simplify them, but I was afraid that in this case the meaning would be distorted. Therefore, I left them as they were.
If there are suggestions for a more competent translation, I will be glad to fix it.
Lithium-ion batteries are high-performance energy storage devices used in many electrical appliances. They can store large amounts of energy in a relatively small volume. Previously it was widely believed that they did not have a memory effect. So experts call the deviation in the operating voltage of the battery, caused by incomplete charging or discharging, as a result of which only part of the stored energy is available, as well as the impossibility of accurately determining the level of battery charge. Scientists from the Paul Scherrer Institute , together with colleagues from the Toyota research laboratory in Japan , have now found that a widely used type of lithium-ion battery has a memory effect. This discovery is particularly important in the use of lithium-ion batteries in the electric vehicle market. The work was published on April 14, 2013 in the scientific journal Nature Materials.
Many of our everyday battery-powered devices are not always smart, as advertised, often have a memory effect. For example, electric shavers or electric toothbrushes, which are charged before they are completely discharged, can be avenged on the user in the future. Batteries remember that you used only part of their capacity - and, in the end, they no longer give out their full energy. Experts call this the "memory effect", which is explained by the fact that the battery's operating voltage drops over time due to incomplete charge-discharge cycles. This means that, although the battery is not yet discharged, the voltage it delivers is sometimes too low to keep the device in working condition. Therefore, the memory effect has two negative consequences: firstly, the useful capacity of the battery decreases, and secondly the correlation between voltage and charge state shifts, so that the latter cannot be reliably determined on the basis of voltage. It has long been known that the memory effect exists in nickel-cadmium and nickel-metal hydride batteries. Since lithium-ion batteries began to be sold successfully in the 1990s, the existence of a memory effect in this type of battery has been ruled out. This new study shows that the view was wrong.

Professor Peter Novak (Petr Novak)
Head of the Electrochemical Energy Storage Section, and co-author of this study. Source: Scanderbeg Sauer Photography.
The effects of the memory effect on electric and hybrid vehicles
The memory effect and abnormalities associated with abnormal operating voltage have already been confirmed on one of the most common materials used as a positive electrode in lithium-ion batteries, lithium-iron phosphate (LiFePO4). With lithium iron phosphate, the voltage remains almost unchanged over a wide range of state of charge. This means that even a small anomaly in the operating voltage can be misinterpreted (as a significant change in charge). Or, to put it differently: when the state of charge is determined by the voltage, a large error may be caused by a slight variation in voltage. The existence of the memory effect is especially relevant when considering the use of lithium-ion batteries in the electric transport sector. In hybrid cars, in particular, the effect may occur due to the many charge / discharge cycles that occur during normal operation. In such vehicles, the battery is partially recharged during engine braking and when operating in generator mode. And in turn, it is discharged, and usually only partially, during the acceleration phase. Numerous successive cycles of partial charging and discharging lead to the addition of separate small memory effects to a larger memory effect, as this new study demonstrates. This leads to an error in estimating the current state of charge of the battery, in the case where the state of charge is calculated based on the current voltage value.
Why the memory effect occurs
Researchers identify the microscopic mechanism that occurs during the charge and discharge process as the main cause of the memory effect in lithium-ion batteries. The electrode material - in this case, iron lithium phosphate (LiFePO4) - consists of a large number of small, micron-sized particles, which are charged and discharged separately one after another. Researchers refer to this charging and discharging model as the "many particles model". Particle charges after particle and includes the release of lithium ions. A fully charged particle does not contain lithium ions (lithium-free) and consists only of iron phosphate (FePO4). The discharge, in turn, involves the re-incorporation of lithium atoms into the particles, so that iron phosphate (FePO4) again becomes iron lithium phosphate (LiFePO 4). Changing the amount of lithium associated with charging and discharging causes a change in the chemical potential of individual particles, which leads to a change in the voltage of the battery. However, charging and discharging are not linear processes. During charging, the chemical potential first increases, with the progressive release of lithium ions. But then, the particles reach the critical lithium content (lithium-content), and, accordingly, the chemical potential. At this point, a sharp transition occurs: the particles discard their remaining lithium ions very quickly, but do not change their chemical potential. This transitional period explains why the battery voltage remains almost unchanged in a wide area (voltage plateau).

Explanation of the schedule number 1
The “memory” effect of a battery occurs in a cycle with a partial charge (in this case, 50 percent of the battery capacity), followed by a complete discharge. At the next charge, the memory effect is expressed as an overvoltage (small “bump”) at the same point at which the previous partial charging cycle ended. For comparison, a graph of the normal stress curve (far right). Source: Nature Publishing Group
Barrier between “rich” and “poor”
The existence of this potential barrier is vital for the manifestation of the memory effect. As soon as the first particles overcame the potential barrier, and became lithium-free, the electrode particles split into two groups. In other words, at present there is a clear distinction between lithium-rich and lithium-poor particles. If the battery is not fully charged, a certain amount of lithium-rich particles that have not crossed the barrier will return. These particles do not remain on the edge of the barrier for a long time, because this state is unstable, and they will “slide down the slope,” that is, their chemical potential will decrease. Even when the battery is discharged again and all the particles will go to the "rest" before the barrier, this division into two groups will be preserved. An important point: during the next charging process, the first group (lithium-poor particles) will overcome the barrier of the first, while the second group (lithium-rich) will “lag behind”. In order for the “deferred” group to overcome the barrier, their chemical potential must be increased, and this is what causes the overvoltage (“bump” on the graph) characterizing the memory effect. Thus, the memory effect is a consequence of the separation of a population of particles into two groups, with very different concentrations of lithium, which follow the "jumping" of particles across a potential barrier, one after the other. This overvoltage, through which the effect is noticeable, is equal to the additional work that must be performed to transport lagging particles through the potential barrier, after a partial charge.

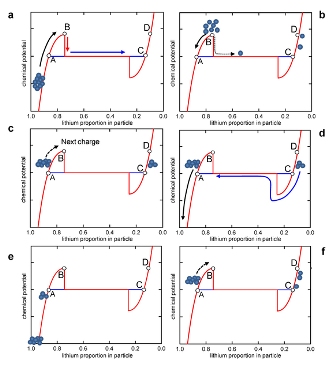
Explanation of schedule number 2
The microscopic mechanism underlying the memory effect follows the Many Particles Model. At first, the chemical potentials of the particles will gradually increase, since the particles emit lithium ions (Fig. B). As soon as they reach point B (chemical potential barrier), the particles begin to abandon the remaining lithium ions, and fully charged (Fig. C). Particles pass through the barrier, one after another, but not all at the same time. After partial charging, some particles return to the front of the barrier (Fig. D). Then these particles “slide down the slope” (slide down the slope), so that the thermodynamic equilibrium is restored. Now, the boundary between the particles "lithium-rich" and "lithium-poor" is set. This separation is maintained, even after the battery is completely discharged (Fig. E and f). During the next charge cycle, this group of lithium-poor particles will cross the barrier. Additional work must be done to move the second “delayed” group of lithium-poor particles through the barrier. This is expressed as overvoltage, which is an indicator of the memory effect. Source: Nature Publishing Group
A pause is needed to eliminate this effect.
The time that passes between charging and discharging the battery plays an important role in determining the state of the battery at the end of these processes. Charging and discharging are processes that change the thermodynamic equilibrium of a battery, and this equilibrium can be achieved after a while. Scientists have discovered that a sufficiently long idle can be used to remove the memory effect. However, according to the multi-particle model, this happens only under certain conditions. The memory effect will disappear only with a sufficiently long break between the partial charge cycles and the subsequent full discharge. In such cases, the groups of particles are still separated after a full discharge, but are on the same side of the potential barrier. Thus, the separation will disappear, since the particles reach an equilibrium state in which they all will have the same lithium content. To prevent the memory effect, you must wait after partial charging and before partial discharge. In this case, the particles will be on opposite sides of the potential barrier, this will prevent their reverse separation into “lithium-rich” and “lithium-poor”.
')
According to Petr Novak, the head of the Electrochemical Energy Storage Section at PSI (Electrochemical Energy Storage Section) and co-author of the publication, the study refutes the well-established misconception: battery powered. These were just suggestions that there would be no similar effect. ” To gain knowledge through research, a combination of reflection and hard work is often fruitful: “Our search results consist of a combination of critical research and careful observation. The effect is actually tiny: the relative voltage deviation is only a few particles per thousand. But the key was the idea of finding it at all. Normal battery tests typically examine complete, rather than partial, charge / discharge cycles.
However, this recent discovery is not the last word for the future use of lithium-ion batteries in automobiles. It is really quite possible that the effect can be detected and will be taken into account through smart software adaptation in battery management systems. If this proves successful, the memory effect will not stand in the way of safe and reliable use of lithium-ion batteries in electric vehicles. So now, engineers are faced with the problem of finding the right treatment with a kind of battery memory.
Text: Leonid Leiva
Following the many particle model described here, it is assumed that charging and discharging the battery occurs particle by particle. In this context, by particles, we mean a kind of "grain". This means that the material (LiFePO4) is not one, but rather consists of a set of granules, the crystal structure of which is the same, but the granules have small differences in size, shape or orientation. This is a typical powder structure. From a technical point of view, they are called "crystallites". It can be imagined as approximately the same cubes in size lying next to each other. Each cube will be slightly rotated relative to its neighbors, that is, the cubes are not strictly aligned, but the crystal structure (hexagon shape) is the same for all.
PS
Thank you Mithgol for an invite.
From the translator: Some sentences are very difficult to understand (when read from the first time), I tried to reformulate and simplify them, but I was afraid that in this case the meaning would be distorted. Therefore, I left them as they were.
If there are suggestions for a more competent translation, I will be glad to fix it.
Source: https://habr.com/ru/post/183476/
All Articles