Tin nanocrystals increase battery power
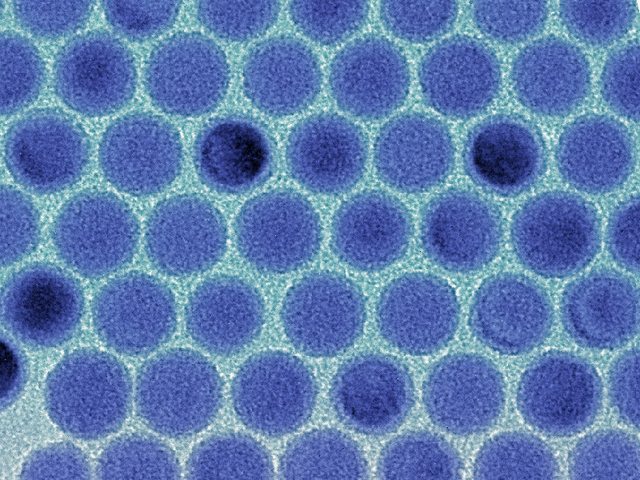
On the photo: monodisperse system of tin particles under an electron microscope.
A group of scientists led by Maxim Kovalenko from the Laboratory of Inorganic Chemistry at the Swiss Higher Technical School of Zurich and the Swiss Federal Laboratory for Materials Science and Technology were able to obtain a new type of nanomaterial that helps to save significantly more energy in the lithium battery.
Nanomaterial consists of tiny tin fragments that are attached to the anode of the battery, its negative pole. The principle of operation is quite simple: when charging a battery, lithium ions are absorbed by the electrode, and during operation and its discharge they are released back. As Kovalenko himself explains, the more ions are absorbed in the process of such “breathing,” the more energy can be stored in the accumulator.
')
The choice of the element for the construction of a nanomaterial was dictated by the fact that each tin atom can absorb up to four lithium ions. Here, however, the problem of physical size arises: a tin crystal increases in size up to three times when ions are absorbed and decreases when they are recovered.
That is why the researchers decided to use nanotechnology to produce tiny tin nanocrystals and embed them into a carbon matrix to produce a porous conductive electrode material. Like a sponge, this material captures and holds lithium ions during charging and releases them during battery operation. If the anode were made of a single piece of tin, a similar effect would not have been observed.
During the development, there were problems in determining the ideal size and the creation of similar, similar to each other nanocrystals. The last question was solved by controlling the temperature and time of the crystal growth phase. The German scientist Kovalenko boasts that his group was the first to create nanocrystals with such precision.
Compared to batteries with conventional electrodes, a double increase in stored energy is promised. Batteries with nanocrystals did not have any particular advantages during the first discharge and charging, but after several cycles of operation, the differences caused by tensine-nanometer crystals on the electrodes became obvious.
At the moment, the optimal size of nanocrystals has been clarified, and now the team wants to achieve knowledge about other most productive components: the best carbon matrix, the best binding substance for the electrodes, the best microscopic structure of the electrodes, the ideal electrolyte. Scientists will also work on the cost of their decisions.
Batteries with much larger capacity can be good news not only for owners and manufacturers of portable consumer electronics, but also for the electric vehicle industry. Relatively lightweight and portable lithium-ion batteries are used today not only in phones, smartphones, laptops, but also in electric cars and motorcycles. In particular, the Department of Energy of the USA is interested in developing more efficient batteries for electric vehicles.
Based on phys.org and Monodisperse and Inorganically Capped Sn and Sn / SnO2 Nanocrystals for High-Performance Li-Ion Battery Anodes .
Source: https://habr.com/ru/post/175953/
All Articles