Do-it-yourself biofuel elements
I just want to warn you that this topic is not exactly on Habr's topic, but in the comments to the post about the element developed at MIT, the idea seemed to be supported, so below I will describe some thoughts about biotolative elements.
The work, on the basis of which this topic was written, was done by me in the 11th grade, and took the second place at the international conference INTEL ISEF.
A fuel cell is a chemical source of current in which the chemical energy of a reducing agent (fuel) and an oxidizer, continuously and separately supplied to the electrodes, is directly converted into electrical energy.
energy. The schematic diagram of the fuel cell (FC) is presented below:
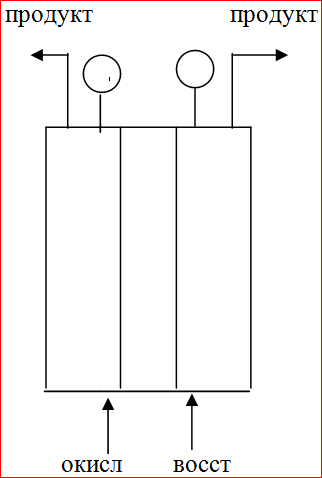
FC consists of an anode, a cathode, an ionic conductor, an anode and a cathode chamber. At the moment, the power of biofuel cells is not enough for use in industrial scales, but BTE with a small capacity can be used for medical purposes as sensitive sensors because the current strength in them is proportional to the amount of fuel processed.
To date, a large number of constructive types of fuel cells have been proposed. In each case, the design of fuel cells depends on the purpose of fuel cells, the type of reagent and the ionic conductor. In a special group emit biofuel elements that use biological catalysts. An important distinguishing feature of biological systems is their ability to selectively oxidize various fuels at low temperatures.
In most cases, immobilized enzymes are used in bioelectrocatalysis, i.e. enzymes isolated from living organisms and fixed on a carrier, but retaining the catalytic activity (partially or completely), which allows them to be reused. Consider the example of a biofuel element in which the enzymatic reaction is coupled with the electrode when using a mediator. Glucose oxidase based biofuel cell layout:
')
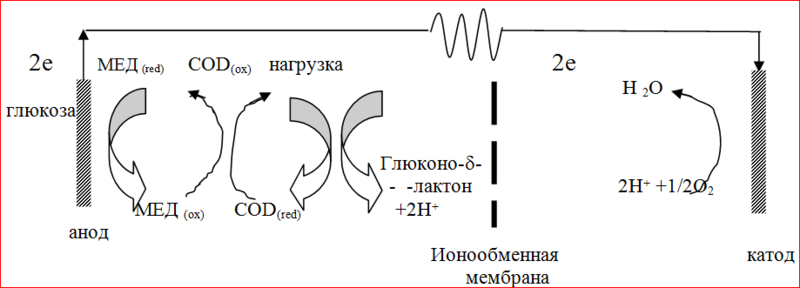
The biofuel element consists of two inert electrodes of gold, platinum or carbon immersed in a buffer solution. The electrodes are separated by an ion-exchange membrane: the anode compartment is blown with air, the cathode - with nitrogen. The membrane allows you to spatially separate the reactions occurring in the electrode compartments of the element, and at the same time provides the exchange of protons between them. Different types of membranes suitable for biosensors are produced in the UK by many firms (VDN, VIROKT).
The introduction of glucose into a biofuel cell containing glucose oxidase and a soluble mediator at 20 ° C leads to a flow of electrons from the enzyme to the anode through the mediator. On the external circuit, electrons go to the cathode, where under ideal conditions in the presence of protons and oxygen water is formed. The resulting current (in the absence of saturation) is proportional to the addition of the rate-determining component (glucose). Measuring stationary currents, it is possible to quickly (5s) determine even small concentrations of glucose - up to 0.1 mM. As a sensor, the described biofuel cell has certain limitations associated with the presence of a mediator and certain requirements for the oxygen cathode and membrane. The latter must retain the enzyme and at the same time pass the low molecular weight components: gas, mediator, substrate. Ion exchange membranes, as a rule, meet these requirements, although their diffusion properties depend on the pH of the buffer solution. Diffusion of components through the membrane leads to a decrease in the efficiency of electron transfer due to side reactions.
To date, there are laboratory models of fuel cells with enzyme catalysts, which by their characteristics do not meet the requirements of their practical application. The main efforts in the next few years will be focused on the refinement of biofuel cells and the further use of the biofuel cell will be associated with a greater degree of medicine, for example: an implantable biofuel cell using oxygen and glucose.
When using enzymes in electrocatalysis, the main problem that needs to be solved is the problem of conjugating an enzymatic reaction with an electrochemical one, that is, ensuring efficient electron transport from the active center of the enzyme to the electrode, which can be achieved in the following ways:
1. The transfer of electrons from the active center of the enzyme to the electrode with the help of a low molecular weight carrier - mediator (mediator bioelectrocatalysis).
2. Direct, direct oxidation and reduction of the active sites of the enzyme at the electrode (direct bioelectrocatalysis).
In this case, the mediator conjugation of the enzymatic and electrochemical reactions, in turn, can be carried out in four ways:
1) the enzyme and the mediator are in the solution volume and the mediator diffuses to the electrode surface;
2) the enzyme is on the surface of the electrode, and the mediator in the volume of the solution;
3) the enzyme and mediator are immobilized on the electrode surface;
4) the mediator is attached to the surface of the electrode, and the enzyme is in solution.
In this paper, laccase served as a catalyst for the cathodic reaction of oxygen reduction, and glucose oxidase (GOD) served as a catalyst for the anodic reaction of glucose oxidation. Enzymes were used in the composition of composite materials, the creation of which is one of the most important stages in the creation of biofuel elements, simultaneously performing the function of an analytical sensor. In this case, biocomposite materials must ensure the selectivity and sensitivity of the determination of the substrate and at the same time have a high bioelectrocatalytic activity approaching the enzymatic one.
Laccase is a Cu-containing oxidoreductase, the main function of which in native conditions is the oxidation of organic substrates (phenols and their derivatives) with oxygen, which is reduced to water. The molecular weight of the enzyme is 40000 g / mol.
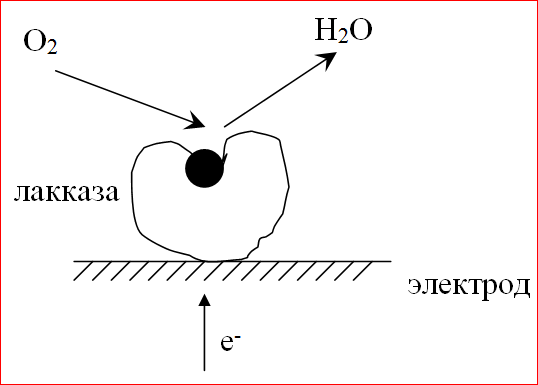
To date, it has been shown that laccase is the most active electrocatalyst for oxygen reduction. In its presence on the electrode in an oxygen atmosphere, a potential close to the equilibrium oxygen potential is established, and the oxygen reduction proceeds directly to the water.
A composite material based on laccase, acetylene black AD-100, and Nafion was used as a catalyst for the cathodic reaction (oxygen reduction). A feature of the composite is the structure that provides orientation of the enzyme molecule with respect to the electron-conducting matrix, which is necessary for direct electron transfer. The specific bioelectrocatalytic activity of laccase in the composite approaches that observed in enzymatic catalysis. The method of conjugation of the enzymatic and electrochemical reaction in the case of laccase, i.e. the method of electron transfer from the substrate through the active center of the laccase enzyme to the electrode is direct bi-electrocatalysis.
Glucose oxidase (GOD), an enzyme of the class of oxidoreductases, has two subunits, each of which has its own active center, (flavivine din dinucleotide) FAD. YEAR is an enzyme selective for the electron donor, glucose, and can use many substrates as electron acceptors. The molecular weight of the enzyme is 180,000 g / mol.
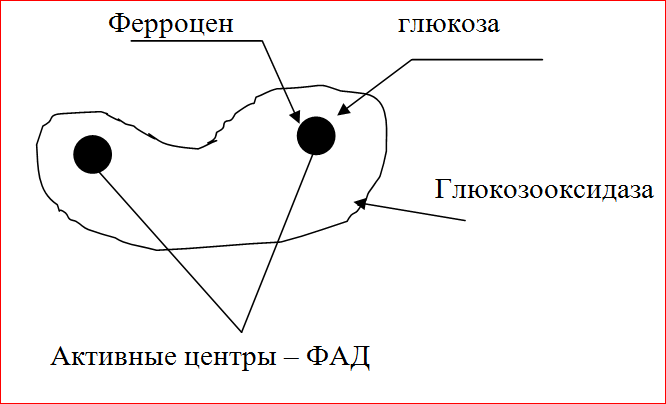
In this work, a composite material based on GOD and ferrocene (FC) was used for anodic oxidation of glucose using a mediator mechanism. Composite material includes YEAR, highly dispersed colloidal graphite (VCG), FC, and Nafion, which made it possible to obtain an electron-conducting matrix with a highly developed surface, to ensure effective transport of reagents to the reaction zone and stable characteristics of the composite material. The method of conjugation of enzymatic and electrochemical reactions, i.e. ensuring efficient electron transport from the active center YEAR to the electrode is a mediator, while the enzyme and the mediator were immobilized on the electrode surface. Ferrocene was used as a mediator, an electron acceptor. During the oxidation of the organic substrate - glucose, ferrocene is reduced and then oxidized on the electrode.
If someone is interested, I can describe in detail the process of obtaining the coating of electrodes, but for this it is better to write in a personal. And in the topic, I just describe the resulting structure.
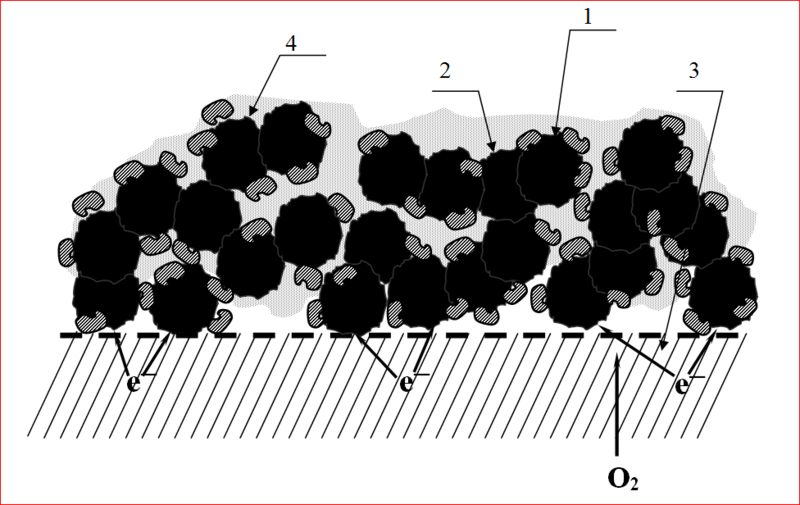
1. AD-100.
2. laccase.
3. hydrophobic porous substrate.
4. Nafion.
After we received the electrodes we went directly to the experimental part. This is how our working cell looked like:
1. Ag / AgCl reference electrode;
2. working electrode;
3. auxiliary electrode - t.
In the experiment with glucose oxidase - purging with argon, with laccase - with oxygen.
The oxygen reduction on soot in the absence of laccase occurs at potentials below zero and occurs in two stages: through the intermediate formation of hydrogen peroxide. The figure shows the polarization curve for the electroreduction of oxygen by laccase immobilized on AD-100, obtained in an oxygen atmosphere in solution with a pH of 4.5. Under these conditions, a stationary potential is established close to the equilibrium oxygen potential (0.76 V). At cathode cathode potentials of 0.76 V, a catalytic reduction of oxygen is observed on the enzyme electrode, which proceeds by direct bioelectrocatalysis directly to the water. In the potential region of cathode 0.55 V, a plateau is observed on the curve, which corresponds to the limiting kinetic current of oxygen reduction. The magnitude of the current limit was about 630 μA / cm2.
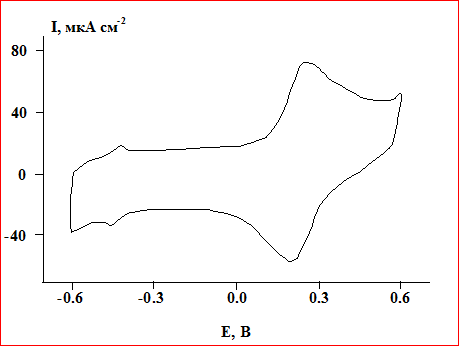
The electrochemical behavior of the composite material, based on the year of Nafion, ferrocene, and VCG, was investigated by cyclic voltammetry (CVA). The state of the composite material in the absence of glucose in the phosphate buffer solution was monitored by charge curves. On the charging curve at a potential (–0.40) V, maxima relating to the redox transformations of the active center of the YEAR - (FAD) are observed, and at 0.20-0.25 V the maxima of oxidation and reduction of ferrocene.
From the obtained results it follows that on the basis of a cathode with laccase, as a catalyst for the oxygen reaction, and an anode based on glucose oxidase for the oxidation of glucose, there is a fundamental possibility of creating a biofuel element. True, there are many obstacles along this path, for example, peaks of enzyme activity are observed at different pH. This led to the need to add an ion-exchange membrane to the BTE. The membrane allows spatially separate reactions occurring in the electrode compartments of an element, and at the same time ensures the exchange of protons between them. Air enters the anode compartment.
The introduction of glucose into a biofuel cell containing a glucose oxidase and a mediator results in a flow of electrons from the enzyme to the anode through the mediator. On the external circuit, electrons go to the cathode, where under ideal conditions in the presence of protons and oxygen water is formed. The resulting current (in the absence of saturation) is proportional to the addition of the rate-determining component, glucose. Measuring stationary currents, it is possible to quickly (5s) determine even small concentrations of glucose - up to 0.1 mM.
Unfortunately, I failed to bring the idea of this BTE to practical implementation, since Immediately after the 11th grade, I went to study as a programmer, and I still work hard today. Thanks to everyone who mastered.
The work, on the basis of which this topic was written, was done by me in the 11th grade, and took the second place at the international conference INTEL ISEF.
A fuel cell is a chemical source of current in which the chemical energy of a reducing agent (fuel) and an oxidizer, continuously and separately supplied to the electrodes, is directly converted into electrical energy.
energy. The schematic diagram of the fuel cell (FC) is presented below:

FC consists of an anode, a cathode, an ionic conductor, an anode and a cathode chamber. At the moment, the power of biofuel cells is not enough for use in industrial scales, but BTE with a small capacity can be used for medical purposes as sensitive sensors because the current strength in them is proportional to the amount of fuel processed.
To date, a large number of constructive types of fuel cells have been proposed. In each case, the design of fuel cells depends on the purpose of fuel cells, the type of reagent and the ionic conductor. In a special group emit biofuel elements that use biological catalysts. An important distinguishing feature of biological systems is their ability to selectively oxidize various fuels at low temperatures.
In most cases, immobilized enzymes are used in bioelectrocatalysis, i.e. enzymes isolated from living organisms and fixed on a carrier, but retaining the catalytic activity (partially or completely), which allows them to be reused. Consider the example of a biofuel element in which the enzymatic reaction is coupled with the electrode when using a mediator. Glucose oxidase based biofuel cell layout:
')

The biofuel element consists of two inert electrodes of gold, platinum or carbon immersed in a buffer solution. The electrodes are separated by an ion-exchange membrane: the anode compartment is blown with air, the cathode - with nitrogen. The membrane allows you to spatially separate the reactions occurring in the electrode compartments of the element, and at the same time provides the exchange of protons between them. Different types of membranes suitable for biosensors are produced in the UK by many firms (VDN, VIROKT).
The introduction of glucose into a biofuel cell containing glucose oxidase and a soluble mediator at 20 ° C leads to a flow of electrons from the enzyme to the anode through the mediator. On the external circuit, electrons go to the cathode, where under ideal conditions in the presence of protons and oxygen water is formed. The resulting current (in the absence of saturation) is proportional to the addition of the rate-determining component (glucose). Measuring stationary currents, it is possible to quickly (5s) determine even small concentrations of glucose - up to 0.1 mM. As a sensor, the described biofuel cell has certain limitations associated with the presence of a mediator and certain requirements for the oxygen cathode and membrane. The latter must retain the enzyme and at the same time pass the low molecular weight components: gas, mediator, substrate. Ion exchange membranes, as a rule, meet these requirements, although their diffusion properties depend on the pH of the buffer solution. Diffusion of components through the membrane leads to a decrease in the efficiency of electron transfer due to side reactions.
To date, there are laboratory models of fuel cells with enzyme catalysts, which by their characteristics do not meet the requirements of their practical application. The main efforts in the next few years will be focused on the refinement of biofuel cells and the further use of the biofuel cell will be associated with a greater degree of medicine, for example: an implantable biofuel cell using oxygen and glucose.
When using enzymes in electrocatalysis, the main problem that needs to be solved is the problem of conjugating an enzymatic reaction with an electrochemical one, that is, ensuring efficient electron transport from the active center of the enzyme to the electrode, which can be achieved in the following ways:
1. The transfer of electrons from the active center of the enzyme to the electrode with the help of a low molecular weight carrier - mediator (mediator bioelectrocatalysis).
2. Direct, direct oxidation and reduction of the active sites of the enzyme at the electrode (direct bioelectrocatalysis).
In this case, the mediator conjugation of the enzymatic and electrochemical reactions, in turn, can be carried out in four ways:
1) the enzyme and the mediator are in the solution volume and the mediator diffuses to the electrode surface;
2) the enzyme is on the surface of the electrode, and the mediator in the volume of the solution;
3) the enzyme and mediator are immobilized on the electrode surface;
4) the mediator is attached to the surface of the electrode, and the enzyme is in solution.
In this paper, laccase served as a catalyst for the cathodic reaction of oxygen reduction, and glucose oxidase (GOD) served as a catalyst for the anodic reaction of glucose oxidation. Enzymes were used in the composition of composite materials, the creation of which is one of the most important stages in the creation of biofuel elements, simultaneously performing the function of an analytical sensor. In this case, biocomposite materials must ensure the selectivity and sensitivity of the determination of the substrate and at the same time have a high bioelectrocatalytic activity approaching the enzymatic one.
Laccase is a Cu-containing oxidoreductase, the main function of which in native conditions is the oxidation of organic substrates (phenols and their derivatives) with oxygen, which is reduced to water. The molecular weight of the enzyme is 40000 g / mol.

To date, it has been shown that laccase is the most active electrocatalyst for oxygen reduction. In its presence on the electrode in an oxygen atmosphere, a potential close to the equilibrium oxygen potential is established, and the oxygen reduction proceeds directly to the water.
A composite material based on laccase, acetylene black AD-100, and Nafion was used as a catalyst for the cathodic reaction (oxygen reduction). A feature of the composite is the structure that provides orientation of the enzyme molecule with respect to the electron-conducting matrix, which is necessary for direct electron transfer. The specific bioelectrocatalytic activity of laccase in the composite approaches that observed in enzymatic catalysis. The method of conjugation of the enzymatic and electrochemical reaction in the case of laccase, i.e. the method of electron transfer from the substrate through the active center of the laccase enzyme to the electrode is direct bi-electrocatalysis.
Glucose oxidase (GOD), an enzyme of the class of oxidoreductases, has two subunits, each of which has its own active center, (flavivine din dinucleotide) FAD. YEAR is an enzyme selective for the electron donor, glucose, and can use many substrates as electron acceptors. The molecular weight of the enzyme is 180,000 g / mol.

In this work, a composite material based on GOD and ferrocene (FC) was used for anodic oxidation of glucose using a mediator mechanism. Composite material includes YEAR, highly dispersed colloidal graphite (VCG), FC, and Nafion, which made it possible to obtain an electron-conducting matrix with a highly developed surface, to ensure effective transport of reagents to the reaction zone and stable characteristics of the composite material. The method of conjugation of enzymatic and electrochemical reactions, i.e. ensuring efficient electron transport from the active center YEAR to the electrode is a mediator, while the enzyme and the mediator were immobilized on the electrode surface. Ferrocene was used as a mediator, an electron acceptor. During the oxidation of the organic substrate - glucose, ferrocene is reduced and then oxidized on the electrode.
If someone is interested, I can describe in detail the process of obtaining the coating of electrodes, but for this it is better to write in a personal. And in the topic, I just describe the resulting structure.

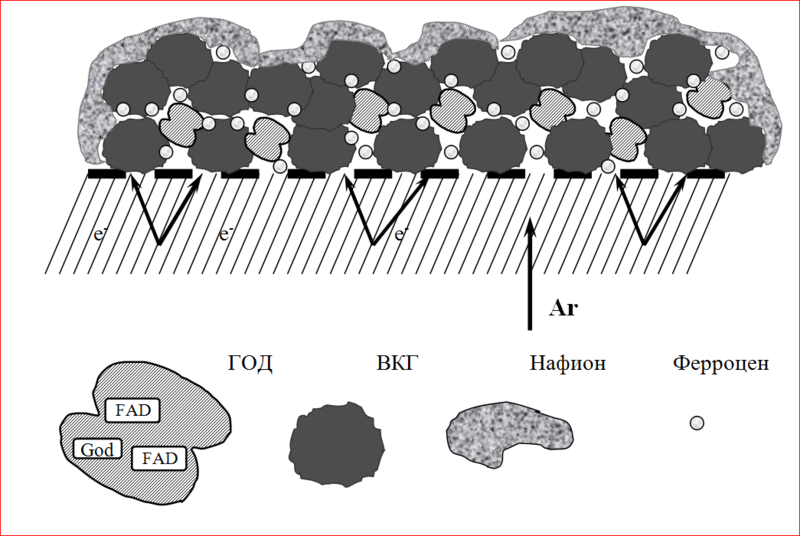
1. AD-100.
2. laccase.
3. hydrophobic porous substrate.
4. Nafion.

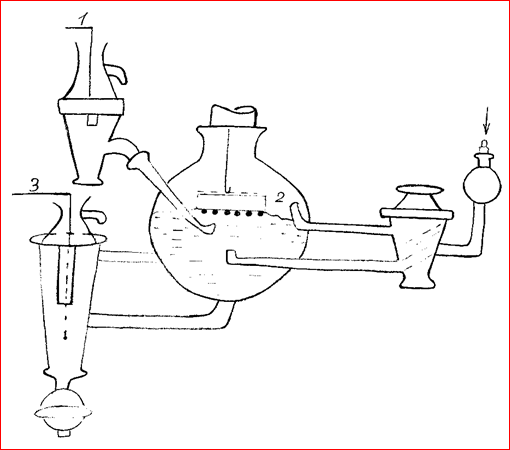
After we received the electrodes we went directly to the experimental part. This is how our working cell looked like:

1. Ag / AgCl reference electrode;
2. working electrode;
3. auxiliary electrode - t.
In the experiment with glucose oxidase - purging with argon, with laccase - with oxygen.
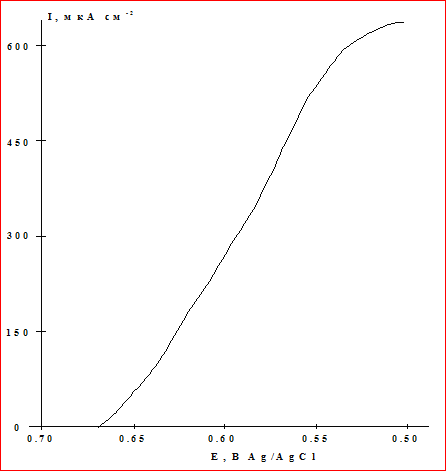
The oxygen reduction on soot in the absence of laccase occurs at potentials below zero and occurs in two stages: through the intermediate formation of hydrogen peroxide. The figure shows the polarization curve for the electroreduction of oxygen by laccase immobilized on AD-100, obtained in an oxygen atmosphere in solution with a pH of 4.5. Under these conditions, a stationary potential is established close to the equilibrium oxygen potential (0.76 V). At cathode cathode potentials of 0.76 V, a catalytic reduction of oxygen is observed on the enzyme electrode, which proceeds by direct bioelectrocatalysis directly to the water. In the potential region of cathode 0.55 V, a plateau is observed on the curve, which corresponds to the limiting kinetic current of oxygen reduction. The magnitude of the current limit was about 630 μA / cm2.

The electrochemical behavior of the composite material, based on the year of Nafion, ferrocene, and VCG, was investigated by cyclic voltammetry (CVA). The state of the composite material in the absence of glucose in the phosphate buffer solution was monitored by charge curves. On the charging curve at a potential (–0.40) V, maxima relating to the redox transformations of the active center of the YEAR - (FAD) are observed, and at 0.20-0.25 V the maxima of oxidation and reduction of ferrocene.

From the obtained results it follows that on the basis of a cathode with laccase, as a catalyst for the oxygen reaction, and an anode based on glucose oxidase for the oxidation of glucose, there is a fundamental possibility of creating a biofuel element. True, there are many obstacles along this path, for example, peaks of enzyme activity are observed at different pH. This led to the need to add an ion-exchange membrane to the BTE. The membrane allows spatially separate reactions occurring in the electrode compartments of an element, and at the same time ensures the exchange of protons between them. Air enters the anode compartment.
The introduction of glucose into a biofuel cell containing a glucose oxidase and a mediator results in a flow of electrons from the enzyme to the anode through the mediator. On the external circuit, electrons go to the cathode, where under ideal conditions in the presence of protons and oxygen water is formed. The resulting current (in the absence of saturation) is proportional to the addition of the rate-determining component, glucose. Measuring stationary currents, it is possible to quickly (5s) determine even small concentrations of glucose - up to 0.1 mM.
Unfortunately, I failed to bring the idea of this BTE to practical implementation, since Immediately after the 11th grade, I went to study as a programmer, and I still work hard today. Thanks to everyone who mastered.
Source: https://habr.com/ru/post/145905/
All Articles