Production control
On the basis of our E1 Euphrates robotic system, it is possible to implement completely different in terms of complexity and scope of application, IT projects.
Not so long ago we received a very unusual and interesting order: to automate the control of the documentation of the industrial process of packaging drugs. There are no ready-made software products for the task set on the entire Russian market!
But for us, nothing is impossible; we have begun to implement this project.
Everything connected with pharmacological production requires increased control over human and automated errors. We have provided a lot of control and reporting points in this business process, have taken into account all the influencing factors to reduce the likelihood of error.
')

Within the framework of the project developed by us, the automated control of the packaging process for drugs includes:
• Creation in the system of a registration and control card (hereinafter referred to as RSC) of the equipment cleaning process
• Establishment of the RCM procedure before packing the finished product
• Stages of packaging. Product dossier
• Performing the packaging and labeling process
• Ad hoc events during the pharmaceutical packaging process
Let us dwell on each of the stages.
Any process of packaging the product begins with cleaning equipment. The replaceable master fills out the RSC page corresponding to this procedure, sets the time and date of cleaning, signs the EDS. The OCC controller assures with its signature the cleanliness of the production line. At this stage, it is possible to form a “Protocol for monitoring the cleaning of the line before production”.
Packaging, as you know, is carried out manually and automatically. For each of these processes, different forms of the protocol and different reporting documents are provided. We divided each of the processes into 11-15 "sub-stages" for the most effective control over each of them. According to the results of the completion of the “sub-step”, an EDS is signed by the employee performing the procedure and the formation of a sheet of the packing protocol in the product file.
Such important files as chemical and pharmaceutical, microbiological quality control, control on bacterial endotoxins and product passport are attached to the packing dossier. As a kind of "bonus" to the dossier at various stages, the requirements are also attached (to receive "in bulk" and materials), the invoice for the delivery of the finished product, the invoice for the return of residues.
All documents are logically interconnected and the quality controller does not waste time searching for the necessary materials. In addition, the output of consolidated reporting on the packaging process also does not take much time: a single button will print the report to print. This eliminates the loss of time and documents, when moving from department to department, reduces the number of errors.
Before starting the packaging of the finished product, a registration and control card (RCM) of the procedure is entered into the system. The data on the final product are entered and controlled into the card throughout the entire production: name of the finished product, series, date of production, expiration date. In addition, there is often a need for additional information, as which information about the manufacturer is entered (if the company manufactures a drug under a franchise), specification data.
Thus, on the title page of the packing protocol, we brought out the basic steps that are automatically filled in using check boxes when filling in the corresponding tab with a replaceable master, or with an OCC controller. This allows you to track at what stage, at the moment, is the process of packing a product: receiving raw materials and supplies, packing in a pack, packing bottles into shipping containers, delivering the finished product to the warehouse, calculating the balance.
By completing the tab on obtaining raw materials, you can go directly to the process itself.
The beginning of the process of packaging and labeling on a labeling machine, cartoning machine and a semi-automatic boxing machine is certified on the appropriate tabs of the file we have already started on the packaging of the product. Control points of production are set quite often: every 15 - 30 minutes, in addition, there is the possibility of selective control every 3 hours. All control information from packaging equipment is transmitted and accounted for in the E1 Euphrates system.
The master sets up the equipment, exposes the data that will mark packs with the product being packed. In order to minimize the influence of the anthropogenic factor (in the form of a master) on skipping the necessary procedures, fixed routes have been established for all its activities.
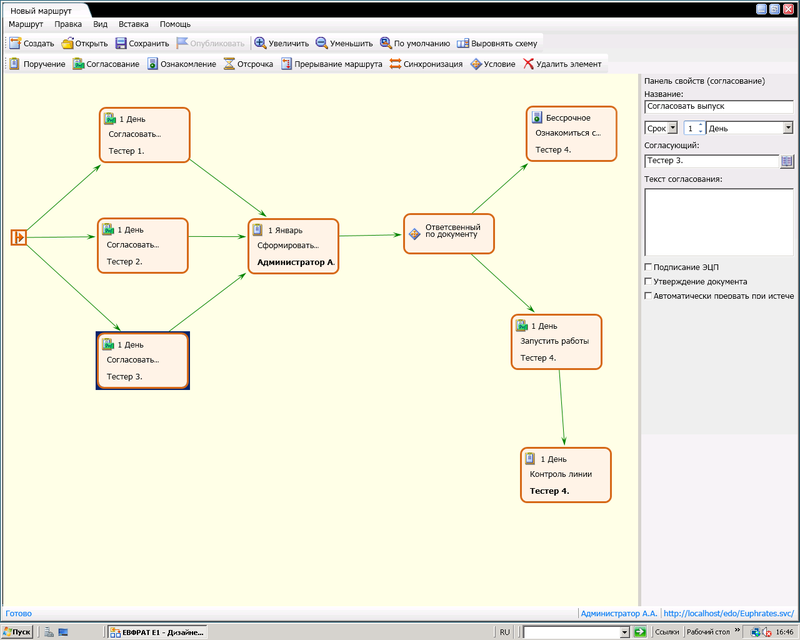
At the control points established for the OCC controller, a visual check is made of the folded instructions and already marked packs and vials (or blisters, depending on the product being packaged). Samples, on request, are attached to the packaging protocol sheets. In the process, the master and the controller fill in the accounting tables of the bottles, self-adhesive labels, and statistics on the issued, used and discarded consumables. The operators fill in the “Material Balance Protocol” in the process of packaging and labeling, which is signed by the OCC controller. Next, the product is packaged in blisters, filled in the necessary protocol tabs, signed by digital signature employees, producing these actions, checkpoints are marked. As the process progresses, the OCC controller fills in the tabs related to monitoring the operation of the equipment, monitoring the correctness and completeness of the attachments to the packet, forms intermediate protocol sheets.
When packaging pharmaceutical products, unregulated events may occur, for example, inconsistency of regulatory documentation during the manufacturing procedure. In this case, the replacement master describes the inconsistency of the regulatory documentation, and the system generates a “Report on packaging incidents”, which is signed by the master and the head of the packing section. Subsequently, this report is certified by the OCC controller. These procedures are provided by routes for handling ad hoc events. Both of these documents fall into the dossier of the product produced, instituted in the E1 Euphrates.
After all these procedures, which look very difficult and incomprehensible in words (although with the help of E1 Euphrates, the process is simplified and accelerated several times than with the traditional method), packaged medications are packed in manufacturing containers and sent to the quarantine zone to the warehouse. And this is where our task is accomplished.
There is nothing impossible for us! The main thing is a competent technical task! :)
Not so long ago we received a very unusual and interesting order: to automate the control of the documentation of the industrial process of packaging drugs. There are no ready-made software products for the task set on the entire Russian market!
But for us, nothing is impossible; we have begun to implement this project.
Everything connected with pharmacological production requires increased control over human and automated errors. We have provided a lot of control and reporting points in this business process, have taken into account all the influencing factors to reduce the likelihood of error.
')

Within the framework of the project developed by us, the automated control of the packaging process for drugs includes:
• Creation in the system of a registration and control card (hereinafter referred to as RSC) of the equipment cleaning process
• Establishment of the RCM procedure before packing the finished product
• Stages of packaging. Product dossier
• Performing the packaging and labeling process
• Ad hoc events during the pharmaceutical packaging process
Let us dwell on each of the stages.
Any process of packaging the product begins with cleaning equipment. The replaceable master fills out the RSC page corresponding to this procedure, sets the time and date of cleaning, signs the EDS. The OCC controller assures with its signature the cleanliness of the production line. At this stage, it is possible to form a “Protocol for monitoring the cleaning of the line before production”.
Packaging, as you know, is carried out manually and automatically. For each of these processes, different forms of the protocol and different reporting documents are provided. We divided each of the processes into 11-15 "sub-stages" for the most effective control over each of them. According to the results of the completion of the “sub-step”, an EDS is signed by the employee performing the procedure and the formation of a sheet of the packing protocol in the product file.
Such important files as chemical and pharmaceutical, microbiological quality control, control on bacterial endotoxins and product passport are attached to the packing dossier. As a kind of "bonus" to the dossier at various stages, the requirements are also attached (to receive "in bulk" and materials), the invoice for the delivery of the finished product, the invoice for the return of residues.
All documents are logically interconnected and the quality controller does not waste time searching for the necessary materials. In addition, the output of consolidated reporting on the packaging process also does not take much time: a single button will print the report to print. This eliminates the loss of time and documents, when moving from department to department, reduces the number of errors.
Before starting the packaging of the finished product, a registration and control card (RCM) of the procedure is entered into the system. The data on the final product are entered and controlled into the card throughout the entire production: name of the finished product, series, date of production, expiration date. In addition, there is often a need for additional information, as which information about the manufacturer is entered (if the company manufactures a drug under a franchise), specification data.
Thus, on the title page of the packing protocol, we brought out the basic steps that are automatically filled in using check boxes when filling in the corresponding tab with a replaceable master, or with an OCC controller. This allows you to track at what stage, at the moment, is the process of packing a product: receiving raw materials and supplies, packing in a pack, packing bottles into shipping containers, delivering the finished product to the warehouse, calculating the balance.
By completing the tab on obtaining raw materials, you can go directly to the process itself.
The beginning of the process of packaging and labeling on a labeling machine, cartoning machine and a semi-automatic boxing machine is certified on the appropriate tabs of the file we have already started on the packaging of the product. Control points of production are set quite often: every 15 - 30 minutes, in addition, there is the possibility of selective control every 3 hours. All control information from packaging equipment is transmitted and accounted for in the E1 Euphrates system.
The master sets up the equipment, exposes the data that will mark packs with the product being packed. In order to minimize the influence of the anthropogenic factor (in the form of a master) on skipping the necessary procedures, fixed routes have been established for all its activities.

At the control points established for the OCC controller, a visual check is made of the folded instructions and already marked packs and vials (or blisters, depending on the product being packaged). Samples, on request, are attached to the packaging protocol sheets. In the process, the master and the controller fill in the accounting tables of the bottles, self-adhesive labels, and statistics on the issued, used and discarded consumables. The operators fill in the “Material Balance Protocol” in the process of packaging and labeling, which is signed by the OCC controller. Next, the product is packaged in blisters, filled in the necessary protocol tabs, signed by digital signature employees, producing these actions, checkpoints are marked. As the process progresses, the OCC controller fills in the tabs related to monitoring the operation of the equipment, monitoring the correctness and completeness of the attachments to the packet, forms intermediate protocol sheets.
When packaging pharmaceutical products, unregulated events may occur, for example, inconsistency of regulatory documentation during the manufacturing procedure. In this case, the replacement master describes the inconsistency of the regulatory documentation, and the system generates a “Report on packaging incidents”, which is signed by the master and the head of the packing section. Subsequently, this report is certified by the OCC controller. These procedures are provided by routes for handling ad hoc events. Both of these documents fall into the dossier of the product produced, instituted in the E1 Euphrates.
After all these procedures, which look very difficult and incomprehensible in words (although with the help of E1 Euphrates, the process is simplified and accelerated several times than with the traditional method), packaged medications are packed in manufacturing containers and sent to the quarantine zone to the warehouse. And this is where our task is accomplished.
There is nothing impossible for us! The main thing is a competent technical task! :)
Source: https://habr.com/ru/post/140422/
All Articles