Bacterial water electrolysis
As you know, hydrogen is considered as one of the most promising energy sources of the future. The only problem is how to effectively get it from water or other organic material.
Scientists from Michigan State University have proposed a new electrochemical process ( PDF ), based on the osmosis process. It is known that if you separate pure water and salt water with a semipermeable membrane, then a weak current will arise due to the movement of ions from the tank with salt water into the container with pure water. The current is so weak that it is not enough for the electrolysis of water, even if you put several membranes one after another.
It is also known that some bacteria are also able to generate a weak current, oxidizing organic materials and getting rid of electrons. But if we consider this process separately, then this current is too small for some industrial application.
American biochemists have suggested combining both methods in one way - constructing a chain of osmotic membranes, and using bacteria at the last anode. It turned out that if bacteria were provided with a sufficient amount of organic material (scientists used acetate), then this “electrical installation” is enough to release hydrogen atoms from water molecules.
')
Scientists have conducted experiments and made sure that hydrogen is produced in a continuous stream until the supply of acetate ends.
The installation efficiency is quite impressive. If you calculate, it turns out that 85% of the energy of combustion of hydrogen is derived from osmotic forces, and only 15% - from fossil fuels. The pumping of water through the system consumes only 1% of the total energy supply, so this installation can easily operate in a constant cycle mode, pumping water on its own and delivering a ready supply of hydrogen. It is only necessary to find a way to automatically supply it with raw materials. For example, it may be waste from the sewage system - bacteria are very illegible in the sense of nutrition. That is, connecting such an osmotic-bacterial module to the sewage system, at the output we will receive a supply of hydrogen and, as a bonus, almost complete destruction of waste.
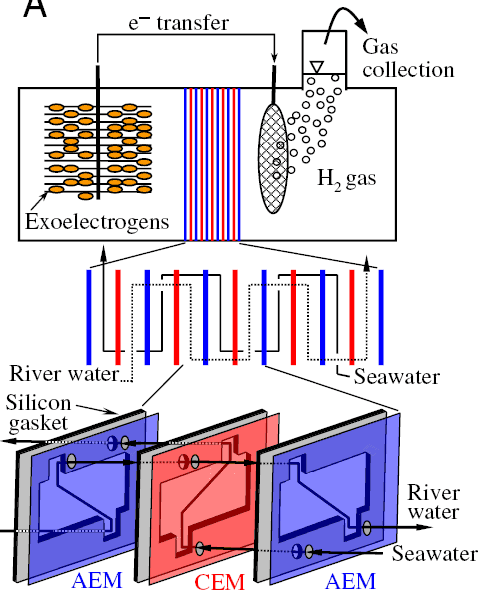
The bad news is that this extremely efficient system requires the use of an expensive platinum-based cathode. The authors of the scientific work tried to use the cathode of a cheaper molybdenum alloy, but the efficiency dropped dramatically. Scientists suggest that in the future it will be possible to find cheap material for the cathode, but so far they have not been able to find such material.

via Ars Technica
Scientists from Michigan State University have proposed a new electrochemical process ( PDF ), based on the osmosis process. It is known that if you separate pure water and salt water with a semipermeable membrane, then a weak current will arise due to the movement of ions from the tank with salt water into the container with pure water. The current is so weak that it is not enough for the electrolysis of water, even if you put several membranes one after another.
It is also known that some bacteria are also able to generate a weak current, oxidizing organic materials and getting rid of electrons. But if we consider this process separately, then this current is too small for some industrial application.
American biochemists have suggested combining both methods in one way - constructing a chain of osmotic membranes, and using bacteria at the last anode. It turned out that if bacteria were provided with a sufficient amount of organic material (scientists used acetate), then this “electrical installation” is enough to release hydrogen atoms from water molecules.
')
Scientists have conducted experiments and made sure that hydrogen is produced in a continuous stream until the supply of acetate ends.
The installation efficiency is quite impressive. If you calculate, it turns out that 85% of the energy of combustion of hydrogen is derived from osmotic forces, and only 15% - from fossil fuels. The pumping of water through the system consumes only 1% of the total energy supply, so this installation can easily operate in a constant cycle mode, pumping water on its own and delivering a ready supply of hydrogen. It is only necessary to find a way to automatically supply it with raw materials. For example, it may be waste from the sewage system - bacteria are very illegible in the sense of nutrition. That is, connecting such an osmotic-bacterial module to the sewage system, at the output we will receive a supply of hydrogen and, as a bonus, almost complete destruction of waste.

The bad news is that this extremely efficient system requires the use of an expensive platinum-based cathode. The authors of the scientific work tried to use the cathode of a cheaper molybdenum alloy, but the efficiency dropped dramatically. Scientists suggest that in the future it will be possible to find cheap material for the cathode, but so far they have not been able to find such material.

via Ars Technica
Source: https://habr.com/ru/post/129255/
All Articles